3.1 Dynamics of Condensed Phase Molecular Systems
Project coordinators: E. T. J. Nibbering , O. KornilovPhase 2 (2011-2017): Transient structure determination of hydrogen-bonded acid-base pairs
The people involved:
Mirabelle Prémont-Schwarz, Maria Ekimova, Benjamin Koeppe, Peter M. Tolstoy, Erik T. J. Nibbering
National and international collaboration: Brian T. Psciuk,#Dequan Xiao,# Victor S. Batista,# Sharon Keinan,† Dina Pines,† Ehud Pines,† Peter M. Tolstoy,ºM. Odelius,* Simon Schreck,+Wilson Quevedo,+Markus Kubin,+Philippe Wernet+, Gül Bekçioğlu,‡ Christoph Allolio,‡ Felix Hoffmann,‡ Daniel Sebastiani‡
†: Department of Chemistry, Ben Gurion University of the Negev, Beer-Sheva, 84105 Israel
#: Department of Chemistry, Yale University, P.O. Box 208107, New Haven, Connecticut 06520-8107, United States of America
º: Department of Chemistry, St. Petersburg University, Russian Federation
*: Department of Physics, Stockholm Universitet, Stockholm, Sweden
+: Ultrafast X-Ray Science Group, Helmholtz-Zentrum Berlin, BESSY II, Albert-Einstein-Str. 15, D-12489 Berlin, Germany
‡: Institut für Chemie, Martin-Luther-Universität Halle-Wittenberg, Von-Danckelmann-Platz 4, 06120 Halle (Saale), Germany.
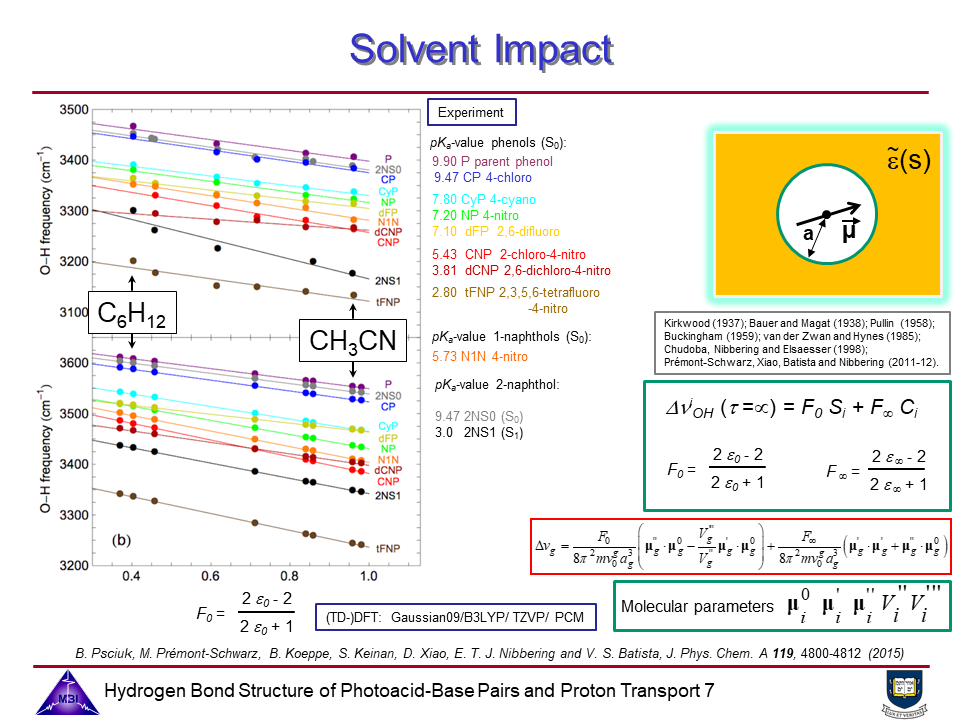
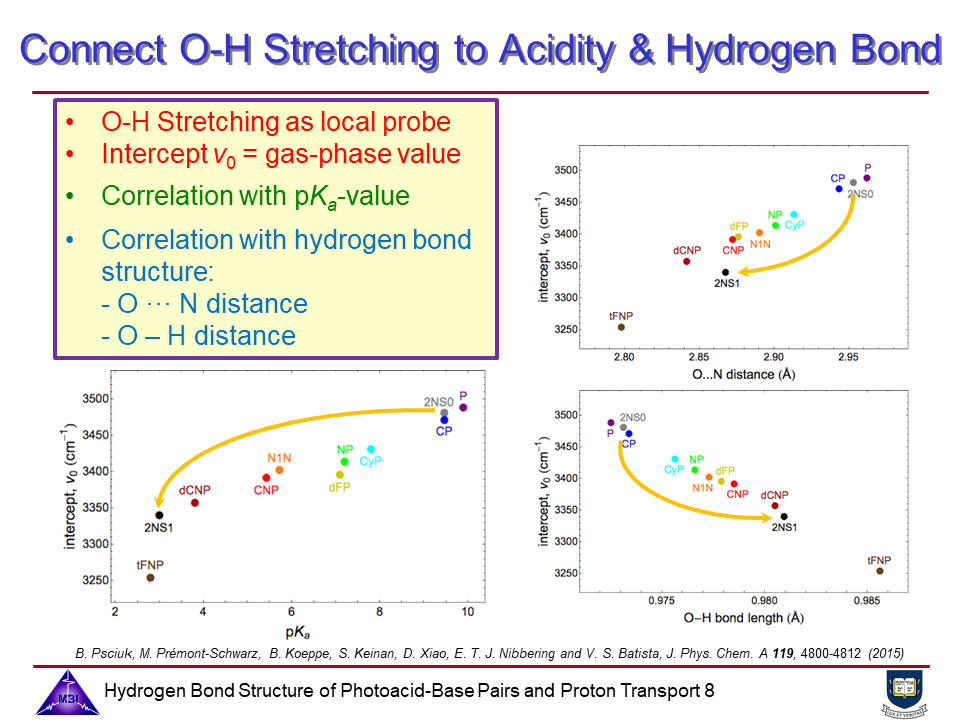
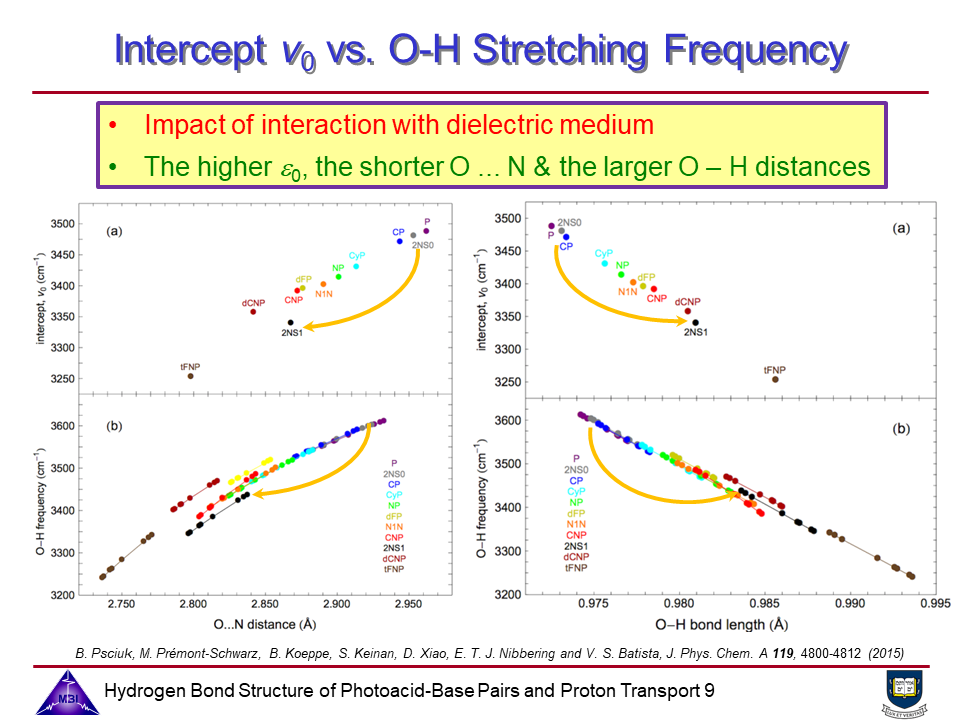
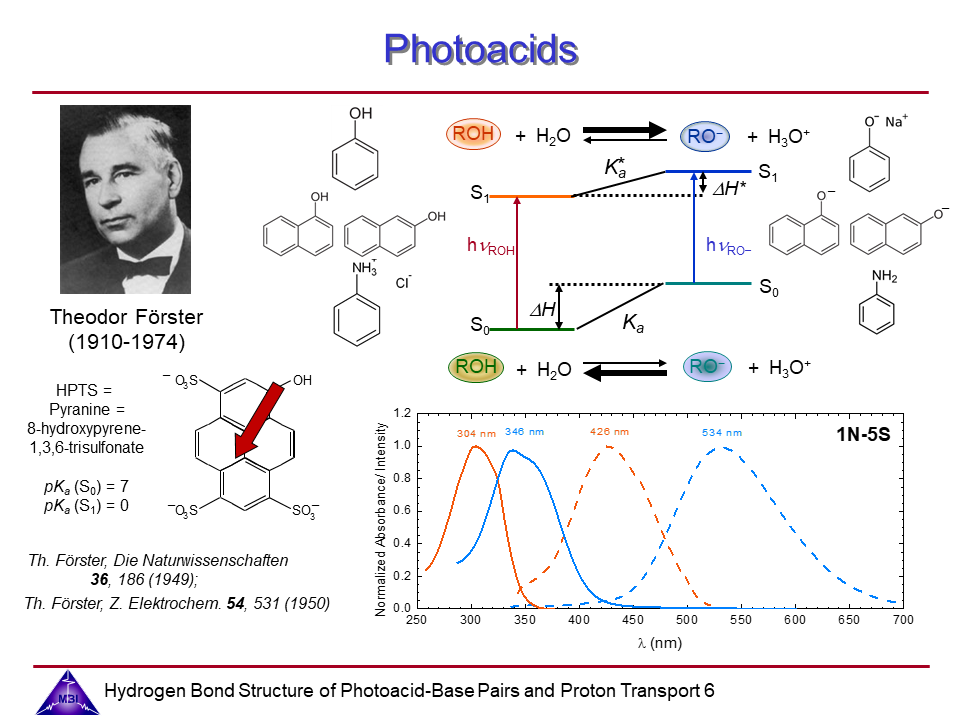
Photoacids are molecular systems that exhibit a pronounced increase in acidity upon electronic excitation, enabling proton transfer dynamics to be studied in real time using time-resolved spectroscopy (see also Phase 1). The goal of the project is to combine the structure resolving potential of time-resolved IR and x-ray spectroscopies to determine hydrogen bond geometries of photoacid-base complexes in liquid solution, and the changes of these hydrogen bond geometries upon electronic excitation of the photoacid chromophores.
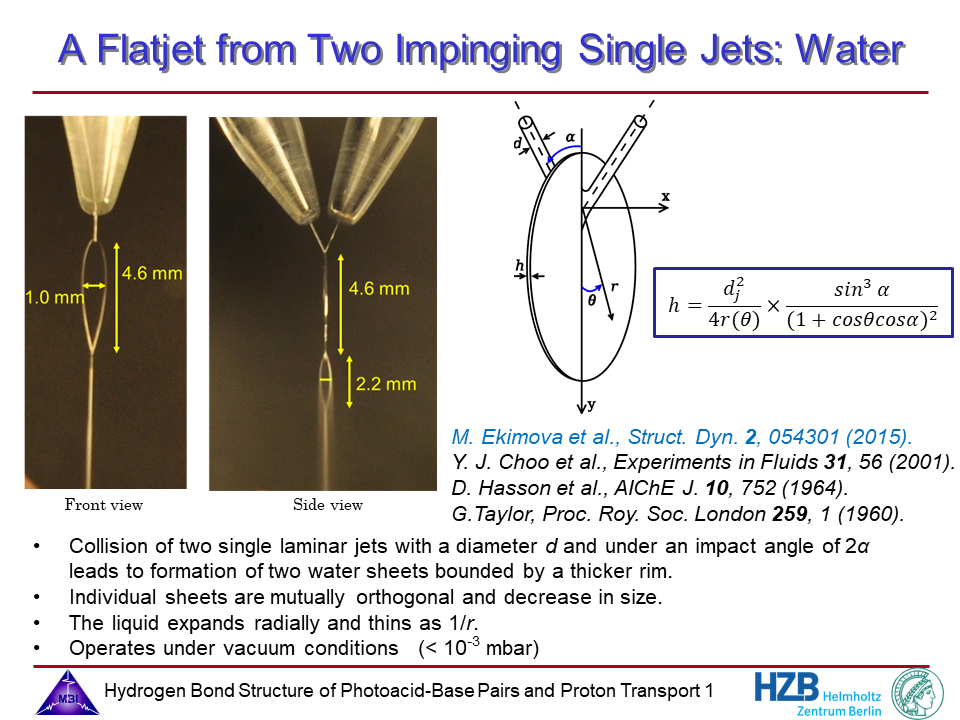
A major step forward into solution phase X-ray absorption spectroscopy in transmission mode has been made in a collaboration with the Wernet and the Odelius groups since 2012. This work has greatly benefitted from the development of a prototype flatjet system. Third party funding comes from the Deutsche Forschungsgemeinsschaft (DFG Normalverfahren NI 492/11-1).
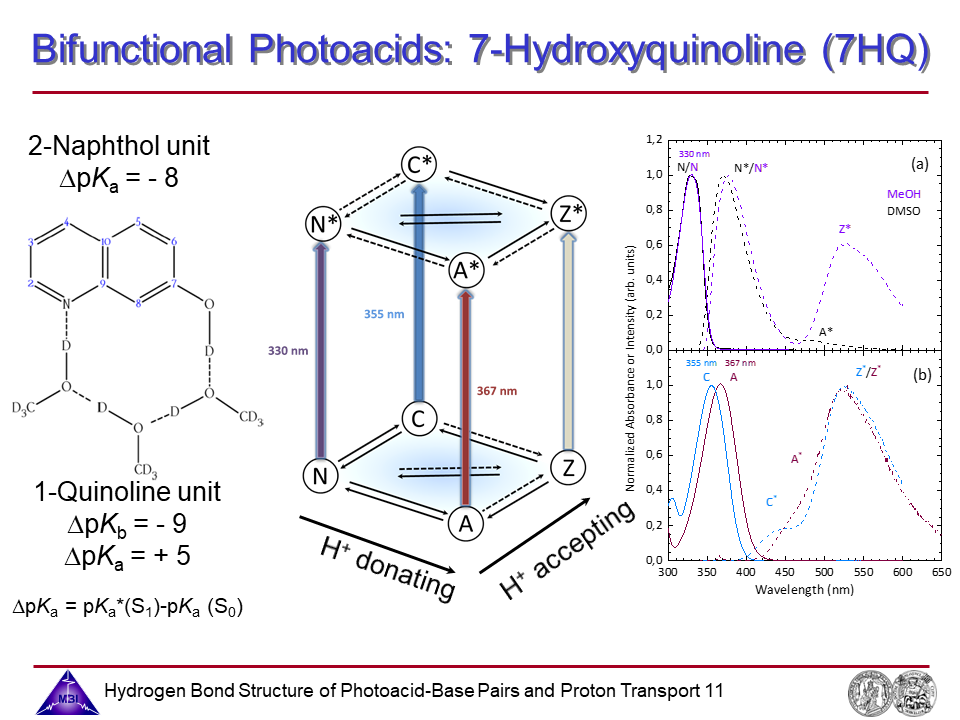
Proton transport along hydrogen-bonded solvent bridges connecting acid and base sites of bifunctional photoacids has been studied in a combined experimental and theoretical approach in a collaboration with the Sebastiani group since 2014. This research is funded by the Deutsche Forschungsgemeinschaft (DFG Normalverfahren NI 492/13-1 / SE 1008/11-1 jointly with D. Sebastiani; 2015-2017).
Hydrogen bond structure of photoacid-base complexes and proton-coupled electron transfer (PCET) has been studied in a combined experimental and theoretical approach jointly with the Batista group (since 2011).
1. Liquid flatjet for use in solution phase soft-x-ray spectroscopy.
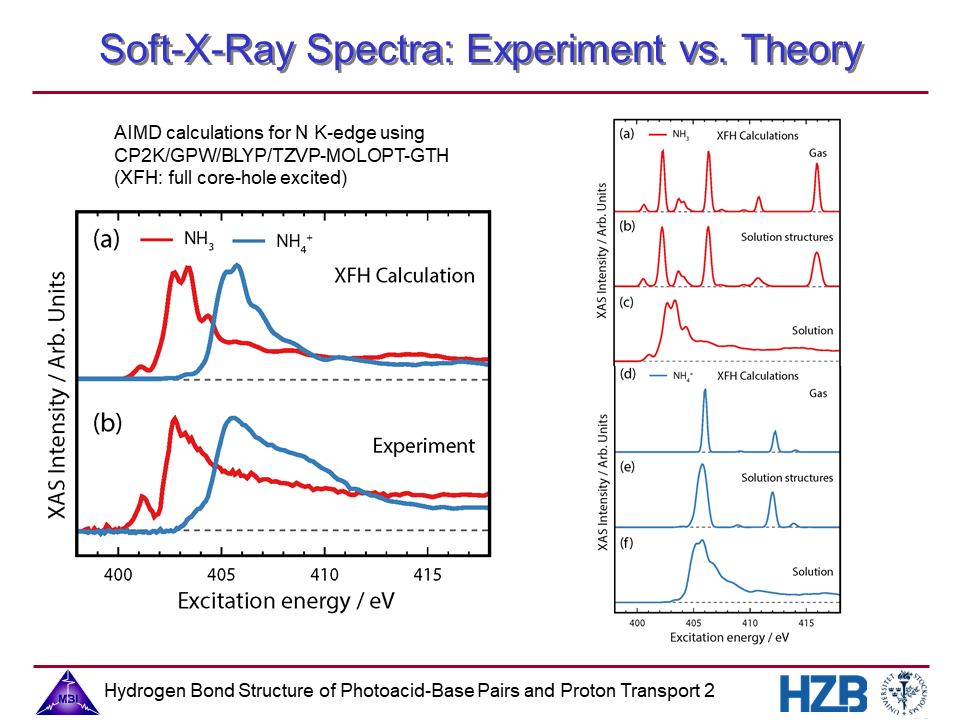
2. Experimental and calculated nitrogen K-edge spectra of NH3 and NH4+.
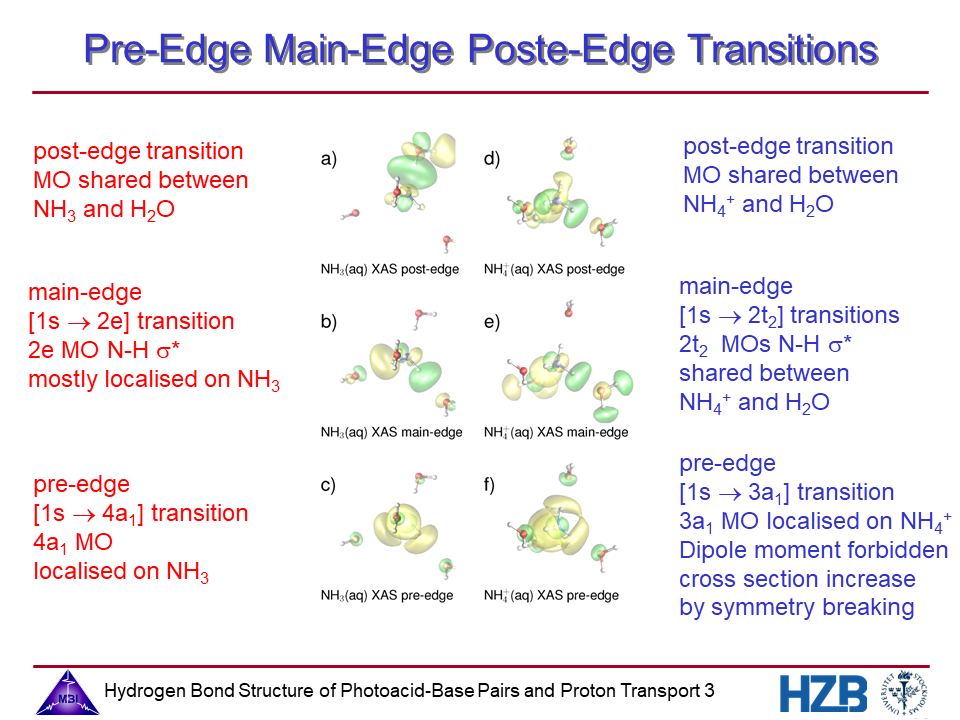
3. Pre-edge main-edge and post-edge transitions for NH3 and NH4+.
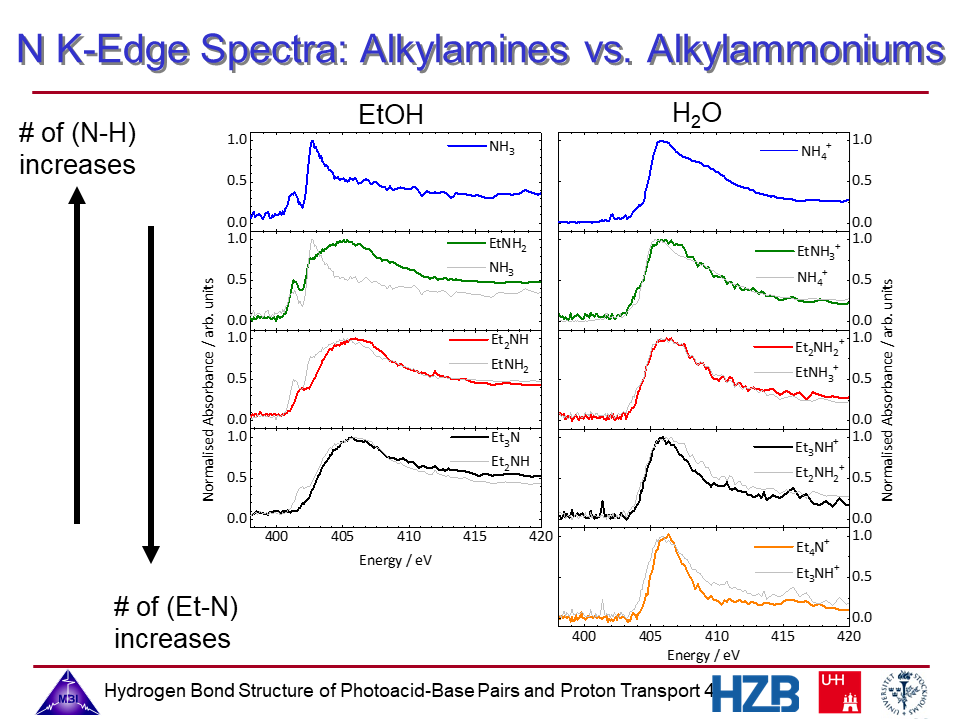
4. Nitrogen K-edge spectra of alkylamines and alkylammonium ions.
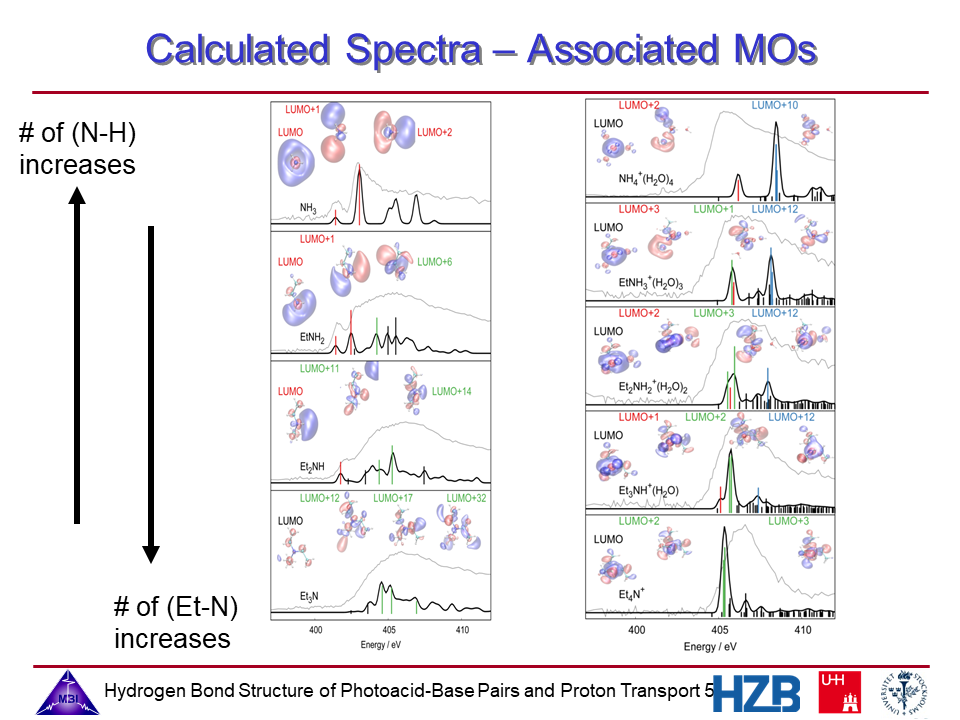
5. Calculated spectra and associated MOs of the alkylamines and alkylammonium ions.
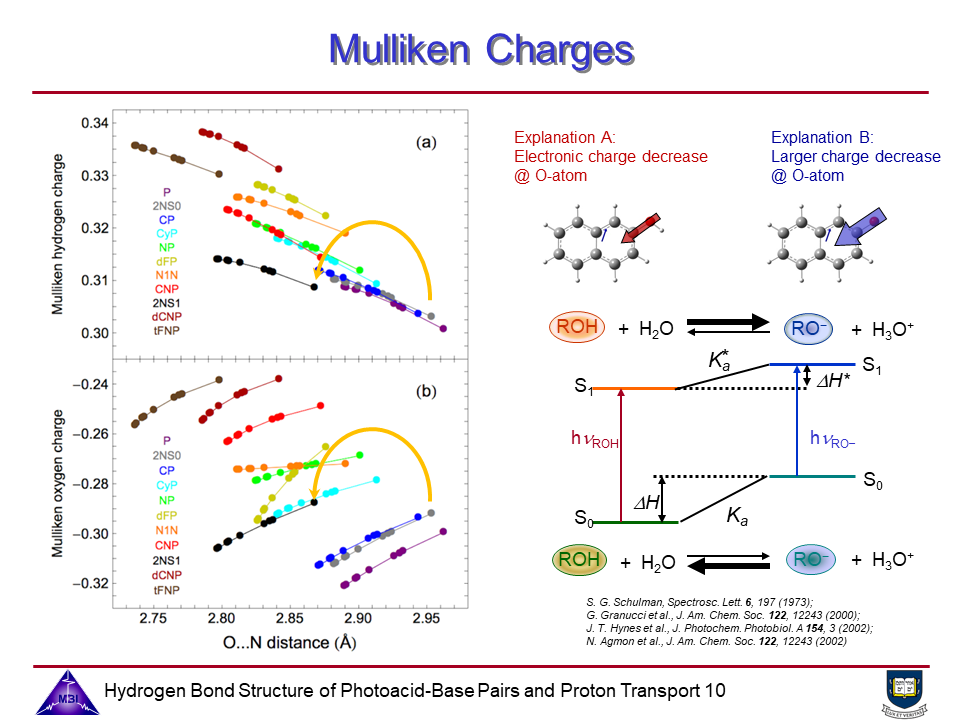
6. Photoacid Förster cycle.
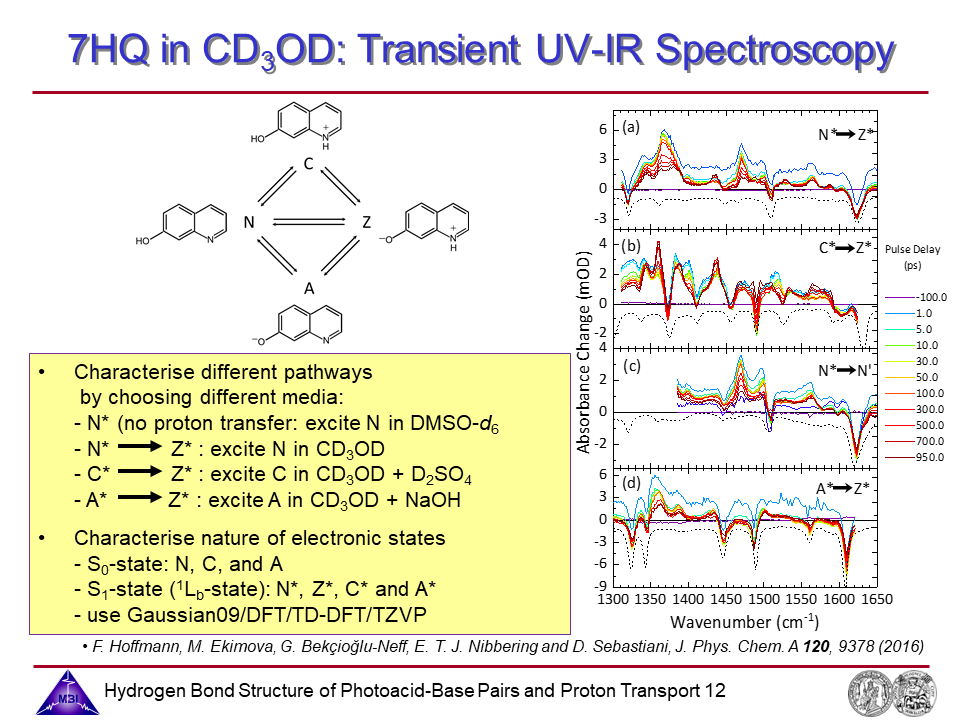
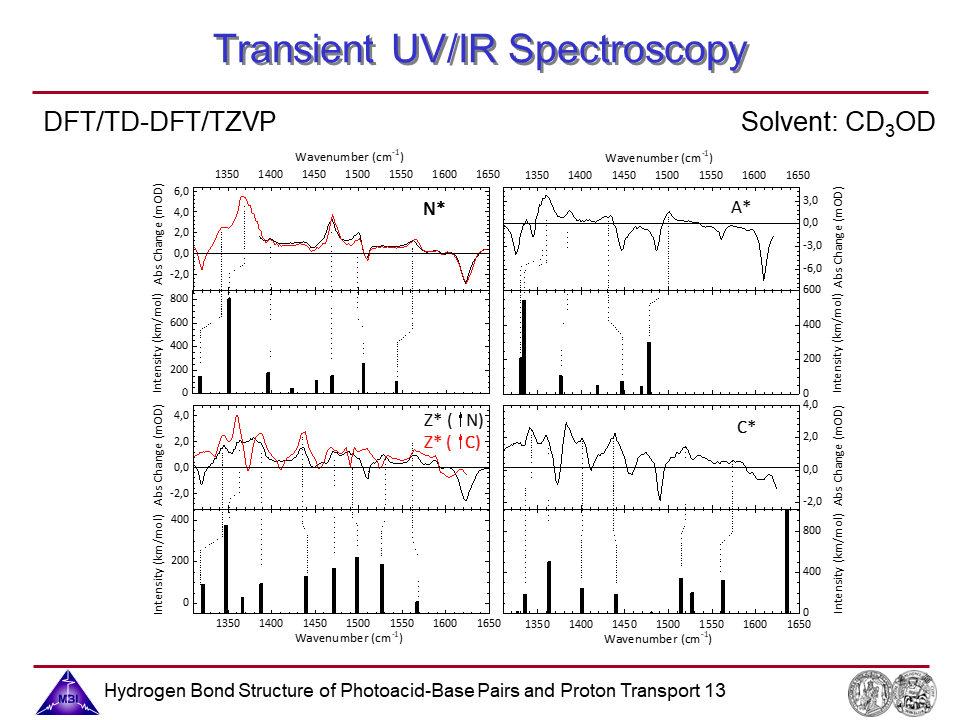
13. Transient UV/IR spectroscopy: Comparison experiment and theory.
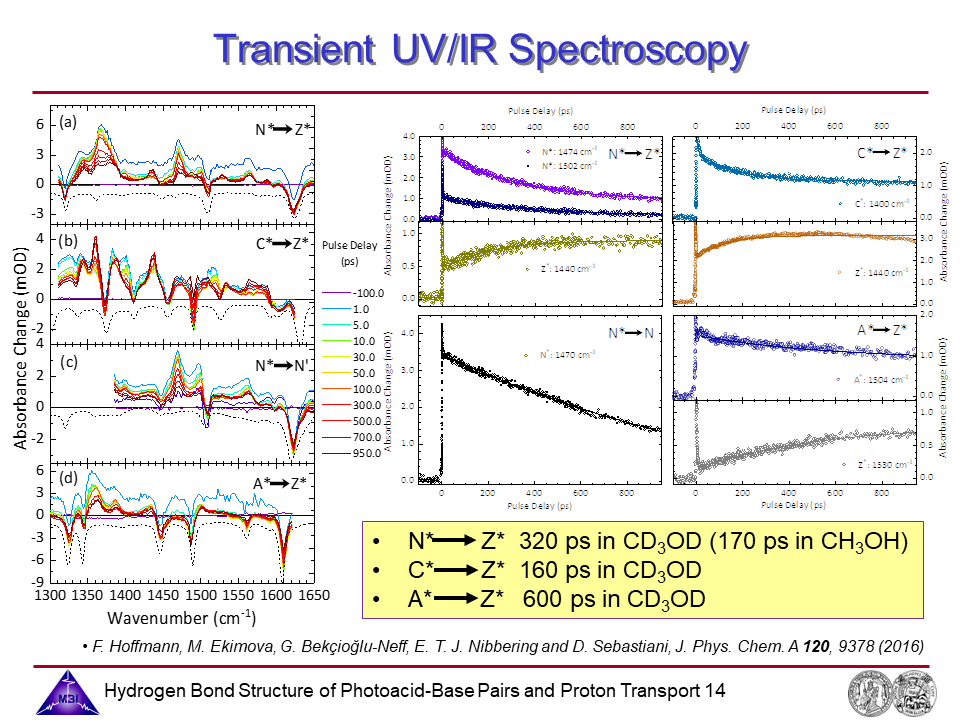
14. Transient UV/IR spectroscopy: spectra and kinetics of neutral tautomer N*, cation C* amd anion A*.
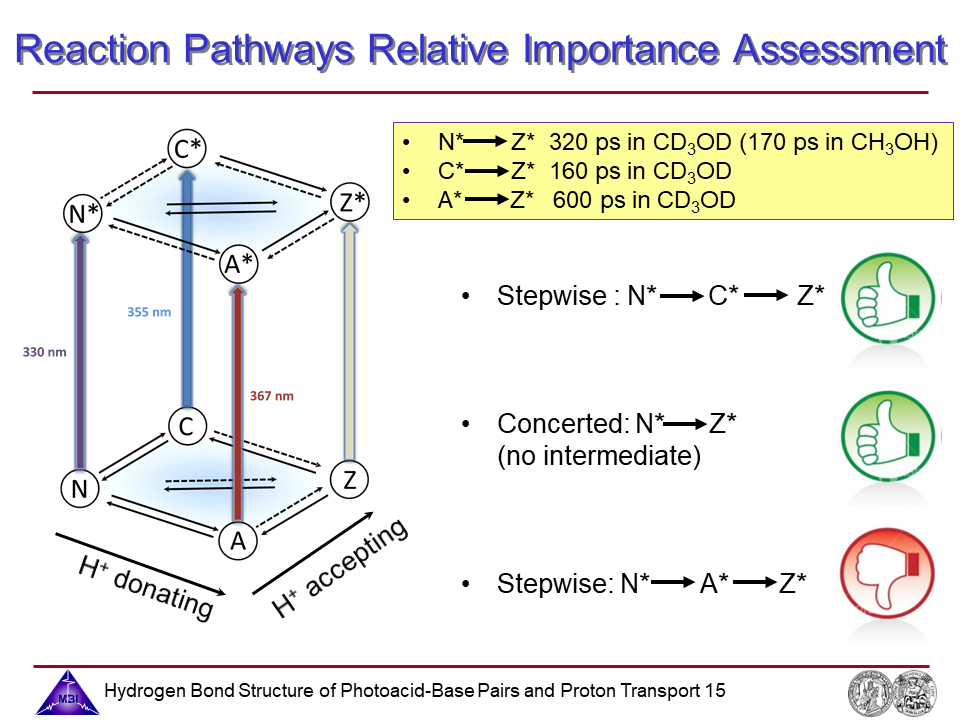
15. Assessment of relative importance of reaction pathways.