3.1 Dynamics of Condensed Phase Molecular Systems
Project coordinators: E. T. J. Nibbering , O. KornilovPhase 1 (2000-2010): Photoacid-Base Neutralization Dynamics
The people involved:
Matteo Rini, Omar F. Mohammed, Katrin Adamczyk, Mirabelle Prémont-Schwarz, Jens Dreyer, Erik T. J. Nibbering
International collaboration: Ben-Zion Magnes,† Dina Pines,† Ehud Pines ,†
†: Department of Chemistry, Ben Gurion University of the Negev, Beer-Sheva, 84105 Israel
Bimolecular proton transfer reactions in liquid solution has been explored since 2000 (more details see link). For either case the interplay between molecular diffusion and reaction dynamics of on-contact complexes has to be taken into account. Femtosecond IR spectroscopy has provided key insight into different stages of aqueous proton transfer between photoacids and bases, taking place in a sequential von Grotthuss-like mechanism. This research was in part funded by: German-Israeli Foundation for Scientific Research GIF-722 jointly with E. Pines (2003-2006) and GIF-876 jointly with E. Pines (2007-2009).
Bimolecular proton transfer in aqueous acid-base neutralization reactions
Bimolecular acid-base neutralization in aqueous solution involves proton transfer reactions between acids and bases with water playing an active role in mediating the exchange of the protons.
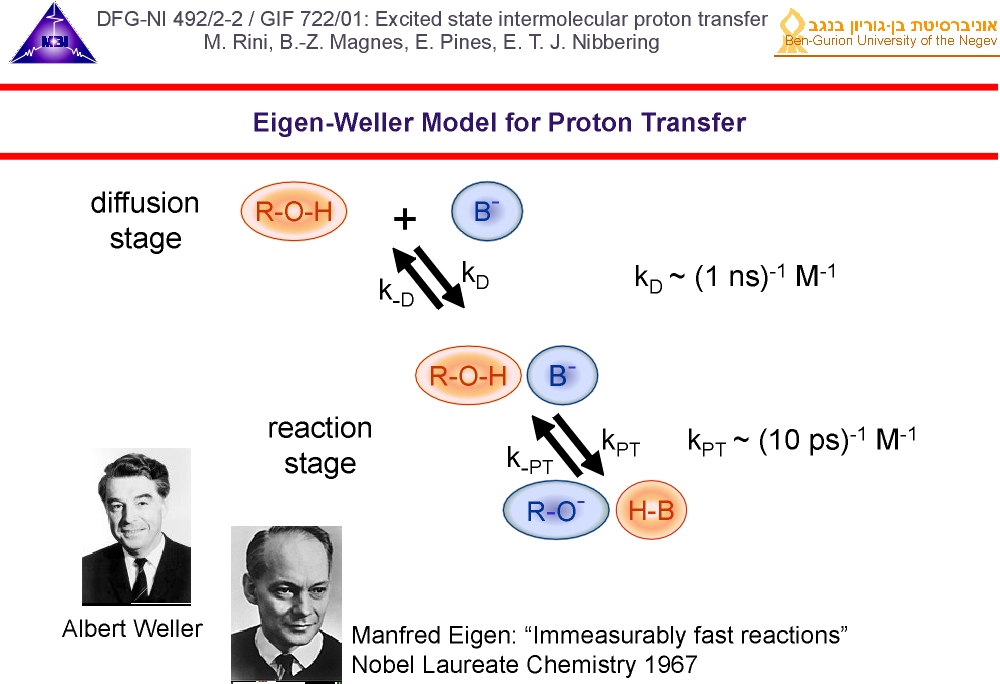
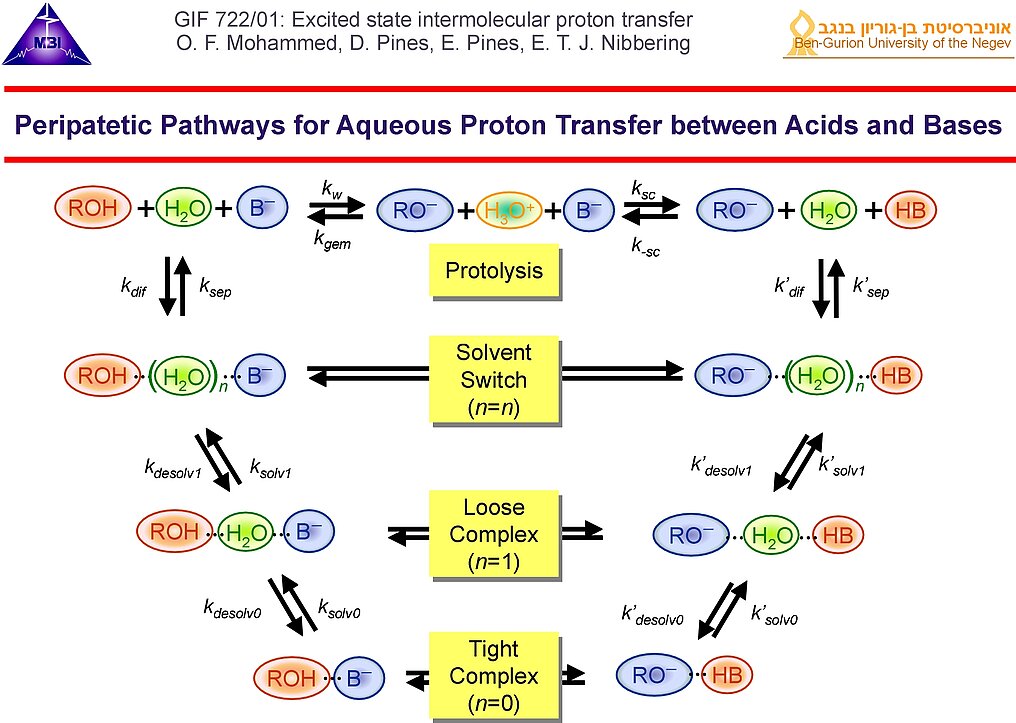
1. Intermolecular proton transfer in condensed phase acid-base neutralization reactions proceed by mutual diffusion of acid and base to each other and subsequent reaction when the acid and base are "on-contact". Since the pioneering work of Weller and Eigen it is known that typical "on-contact" reaction rates typically lie in the picosecond range.
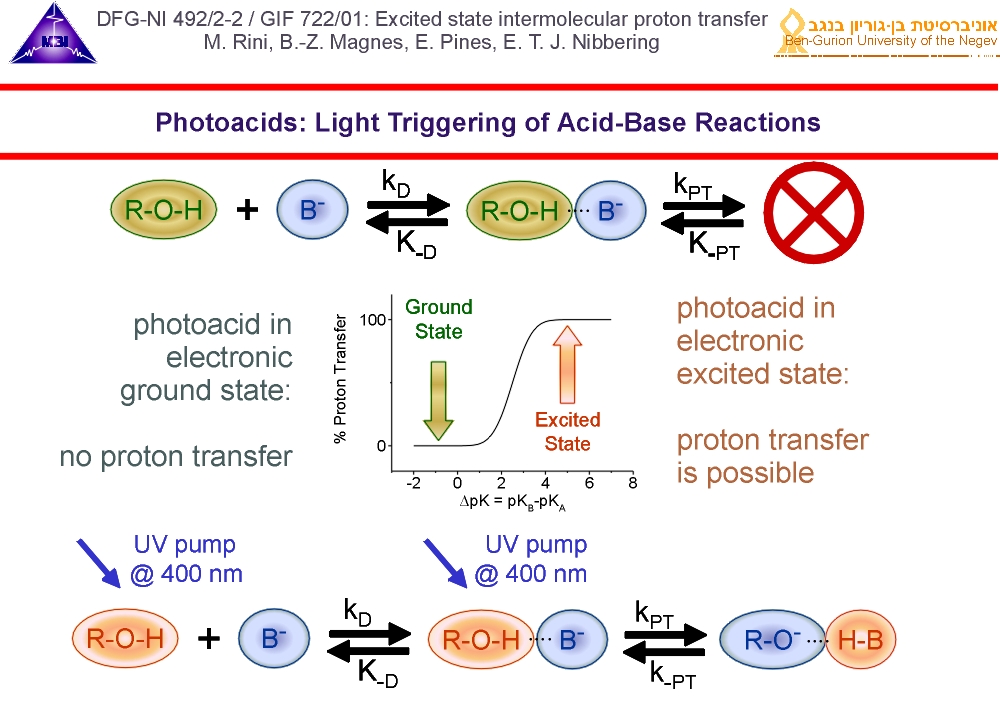
2. Photoacids are ideal systems to study the proton transfer dynamics in real-time. When in the electronic ground state proton transfer does not take place when the difference between the pKa-values of the conjugate acid HB and the photoacid R-OH is smaller than 2. When photo-excited, the pKa -value of the photoacid drops with values between 5-10. Proton transfer is then made possible by the optical trigger pulse.
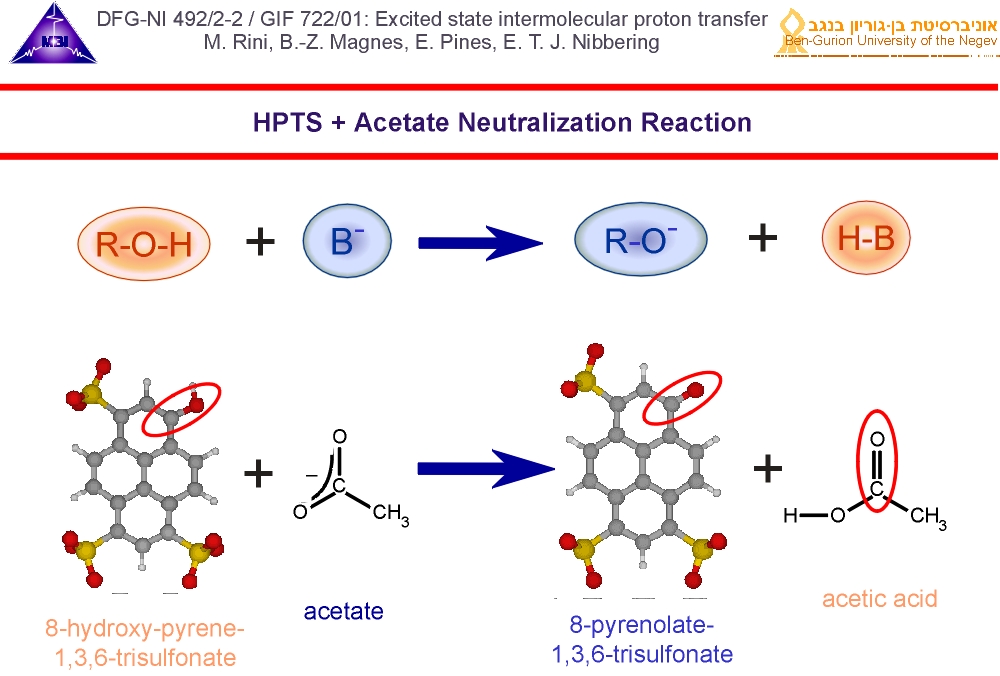
3. We have used 8-hydroxy-pyrene-1,3,6-trisulfonate (HPTS) as photoacid and acetate as base., dissolved in deuterated water. We excite the photoacid with a 400 nm pump pulse, and follow the dynamics with an IR-pulse tuned to marker modes of the photoacid, of the conjugate photobase and of acetic acid, that is formed when acetate accepts a deuteron. We thus follow the reaction two sides: the photoacid/photobase (telling us when the deuteron leaves the photoacid) and the product acetic acid (telling us when the deuteron arrives at the base acetate).
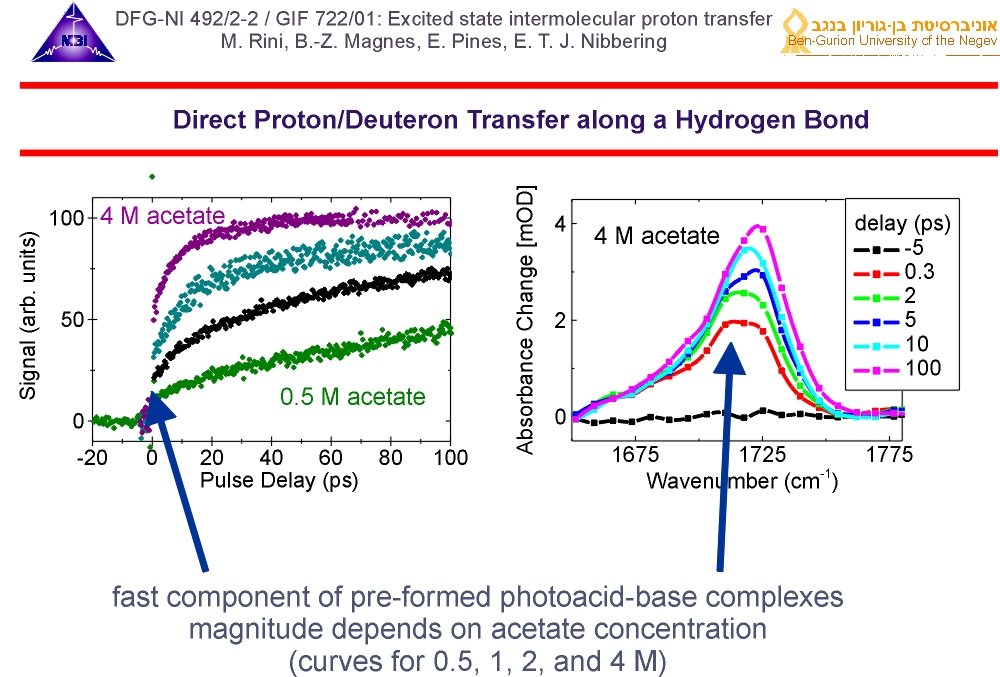
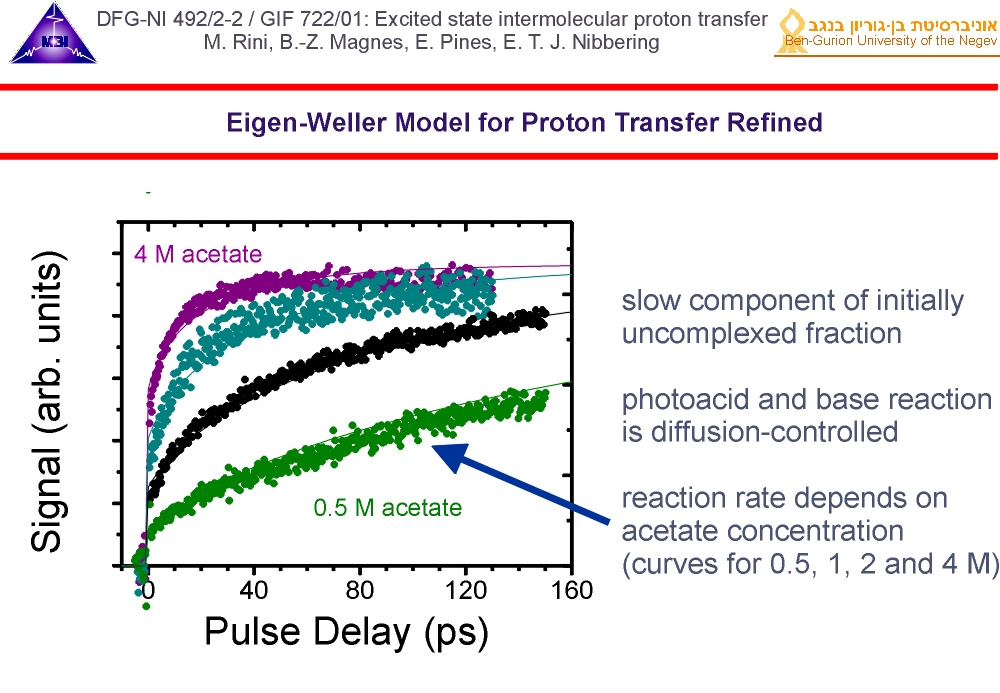
4. At high concentrations of acetate we observe a fast component, that is due to pre-formed photoacid-base complexes. In these complexes the photoacid and base are in direct contact through a hydrogen-bond, along which the reaction can proceed rapidly. The deuteron transfer is found to be faster than our time resolution of 150 fs.
5. For the fraction of photoacid molecules that are initially uncomplexed when they are excited by the optical pump pulse, the reaction dynamics with the base acetate is diffusion-controlled. Modelling this diffusion-controlled reaction (solid lines) tells us that the "on-contact" reaction dynamics occurs on picosecond time scales.
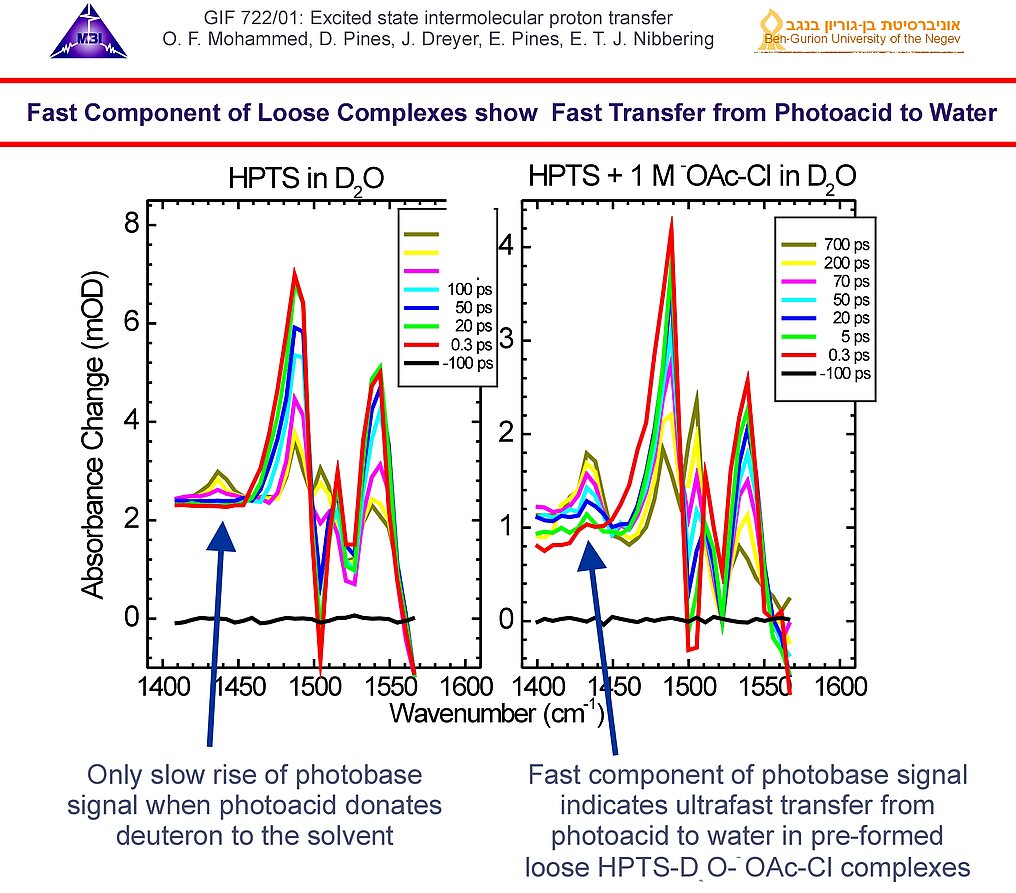
6. In the case of the deuteron transfer reaction of HPTS in 1 M potassium chloroacetate (KOAc-Cl) 20% of the photoacid releases the deuteron within time resolution (< 150 fs), as evidenced by the initial rise of the 1435 cm-1 photobase band. Comparison with the case of HPTS in D2O (no base added) reveals that in this case the signal is generated in the "loose" HPTS--D2O--OAcCl complexes when the deuteron is released to the water bridge.
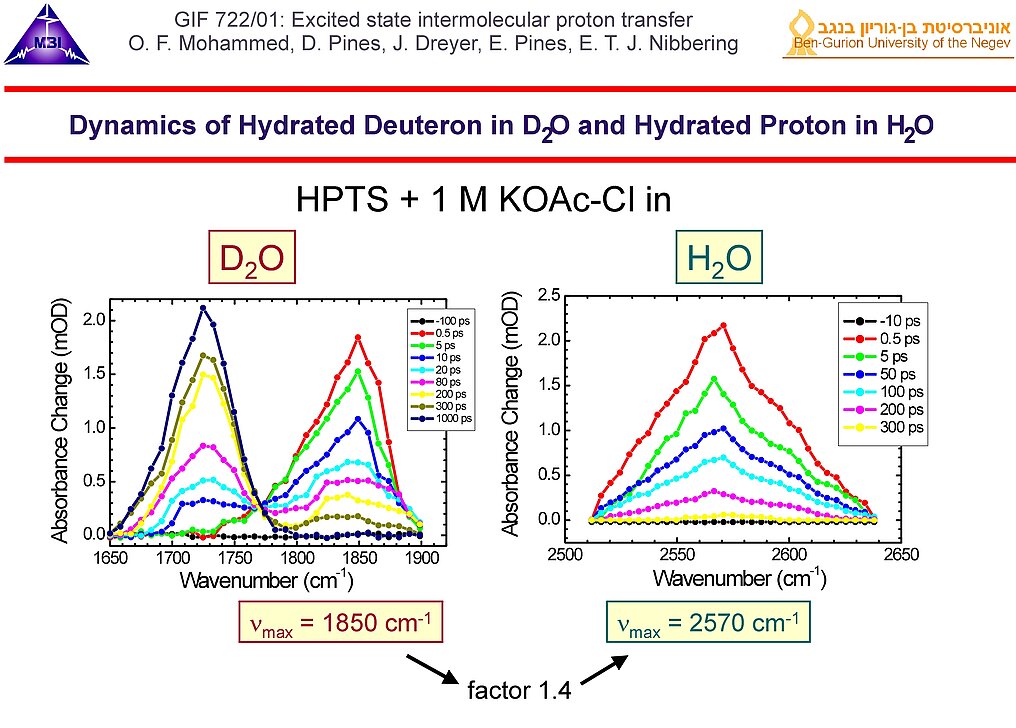
7. Comparison of the dynamics of the hydrated deuteron and hydrated proton bands measured for HPTS in 1 M KOAc-Cl in D2O and H2O, respectively. At longer pulse delays the deuteron/proton is transferred to the base, as indicated by the decay of the hydrated deuteron/hydrated proton bands and the rise of the C=O stretching band of DOAc-Cl.
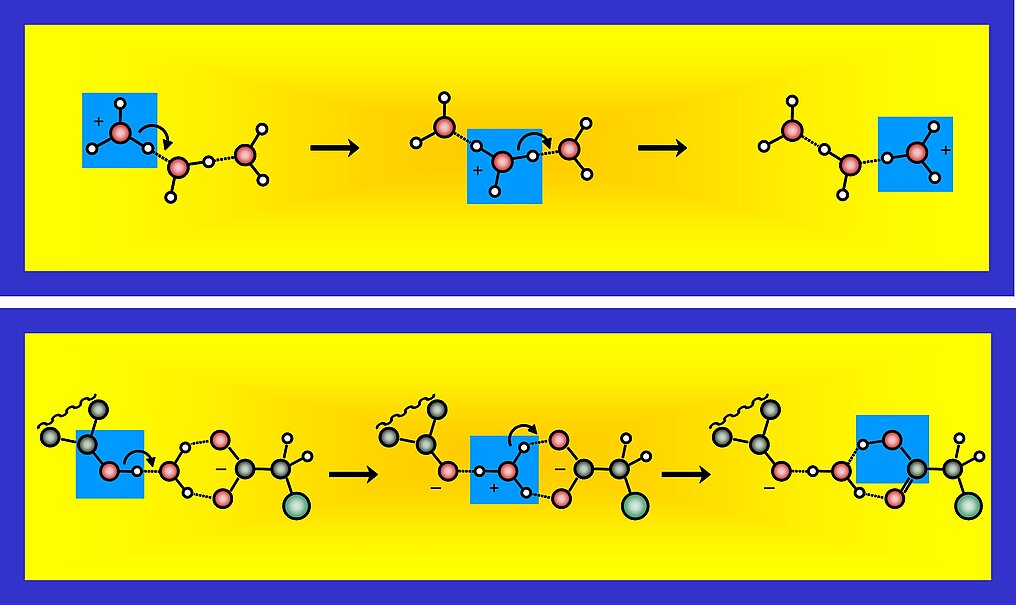
9. Several proton transfer pathways between acids and bases having different number of water molecules as bridge can be considered. Because proton transfer reactions are typically reversible, many peripatetic pathways may play a key role in the solution phase dynamics. The phrase "peripatetic" in this context has been used first by Casey Hynes, see his News & Views commentary published in 2007 on this.
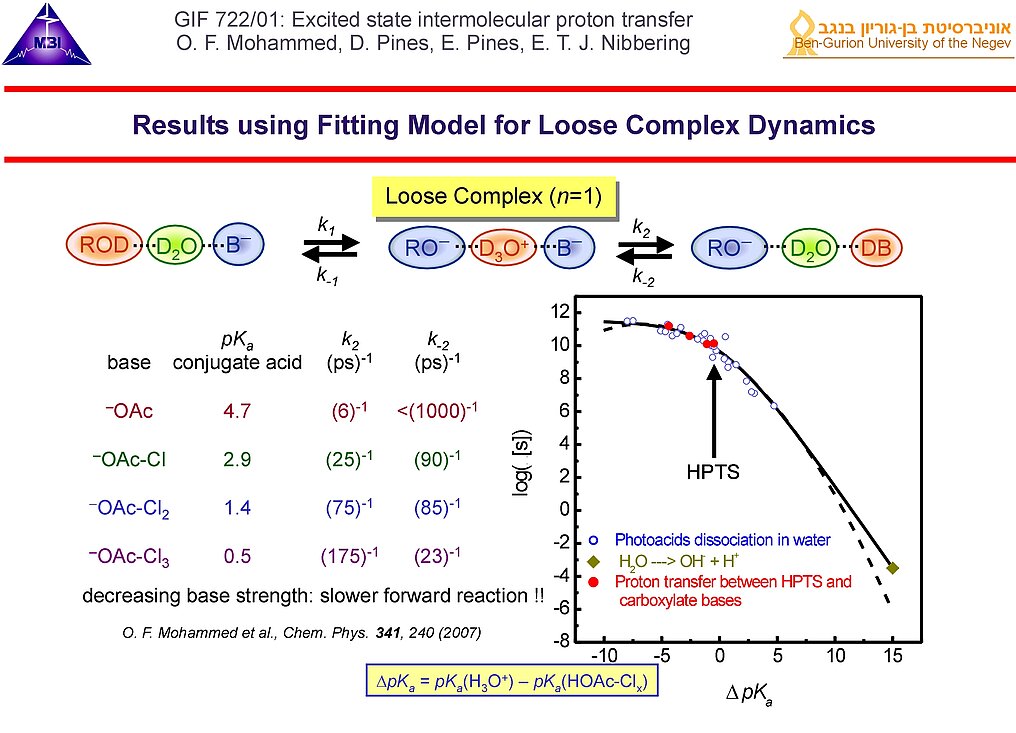
10. An example from the analysis of the broad data set obtained on the photoinduced reaction between HPTS and different carboxylate bases is shown here. For the loose complex pathway (only a single water molecule bridging photoacid and base) it follows that for the second proton transfer step the forward rate diminishes as function of basicity of the base (and the backward rate increases). As such the equilibrium shifts from the right hand side when using acetate to the centre when using trichloroacetate. The proton transfer reaction rate observed in the current experiment (red dots) follow the general trend found for proton dissociation of a large class of photoacid molecules (open blue circles), obeying a Marcus-type free energy correlation for proton transfer in aqueous conditions. This points to an active role the solvent water plays in such reactions.
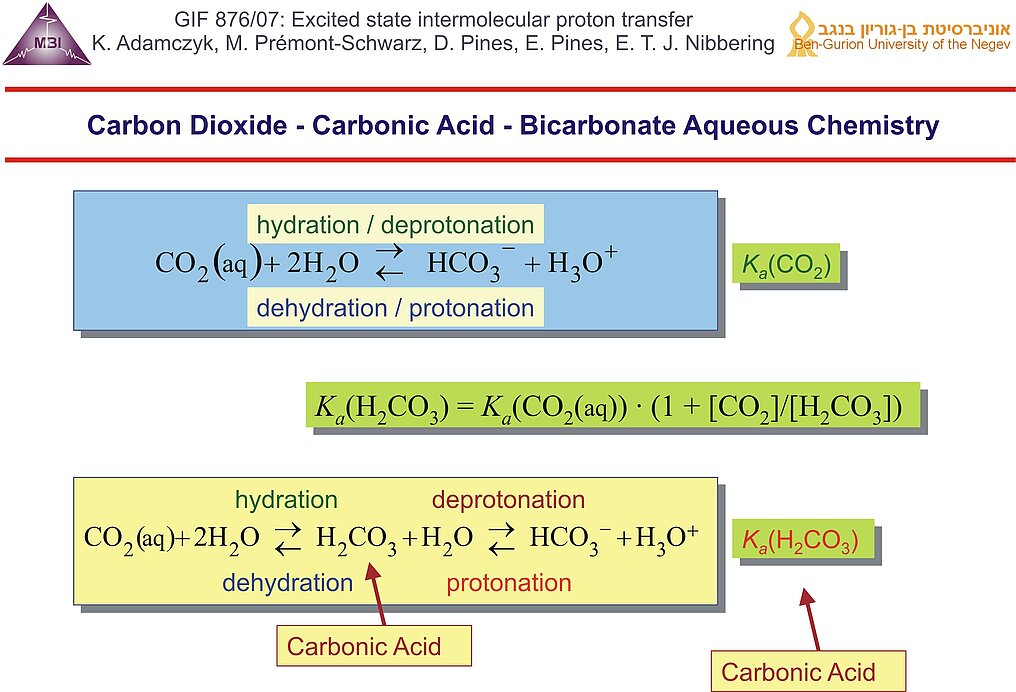
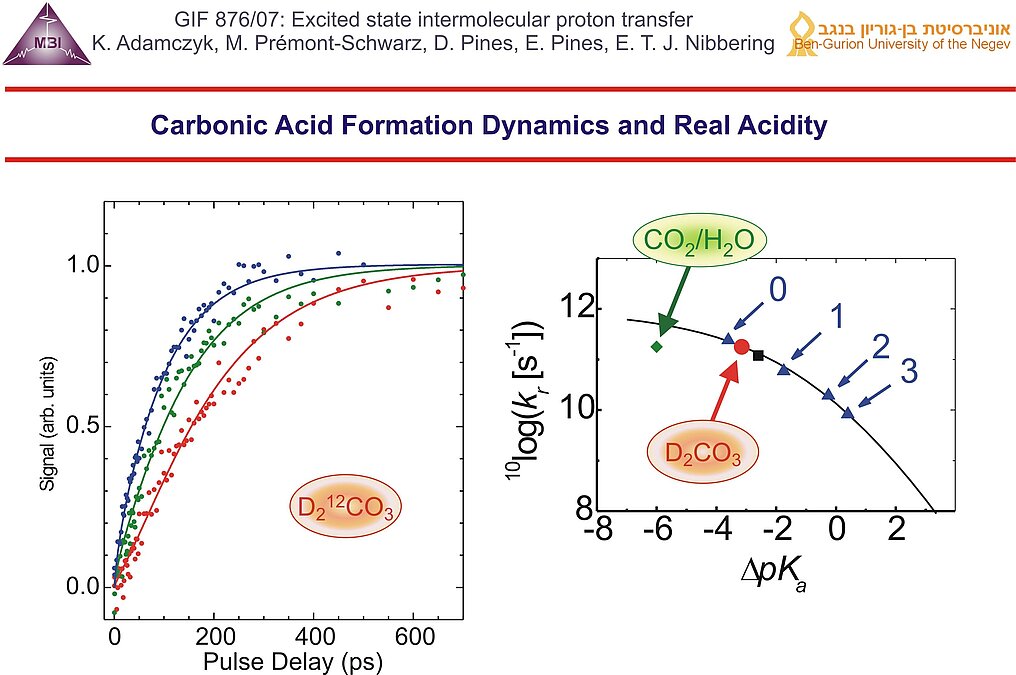
11. Aqueous carbon dioxide chemistry is usually described using an equilibrium between CO2/H2O and HCO3-/H3O+, with the effective acid dissociation constant Ka(CO2) for which pKa = 6.35. In reality the hydration/dehydration and deprotonation/protonation steps have to be treated separately, with carbonic acid as intermediate. Carbonic acid has not been directly observed in aqueous solution, and as a result the real acidity of carbonic acid has only been roughly estimated to be pKa ~ 3.6 .
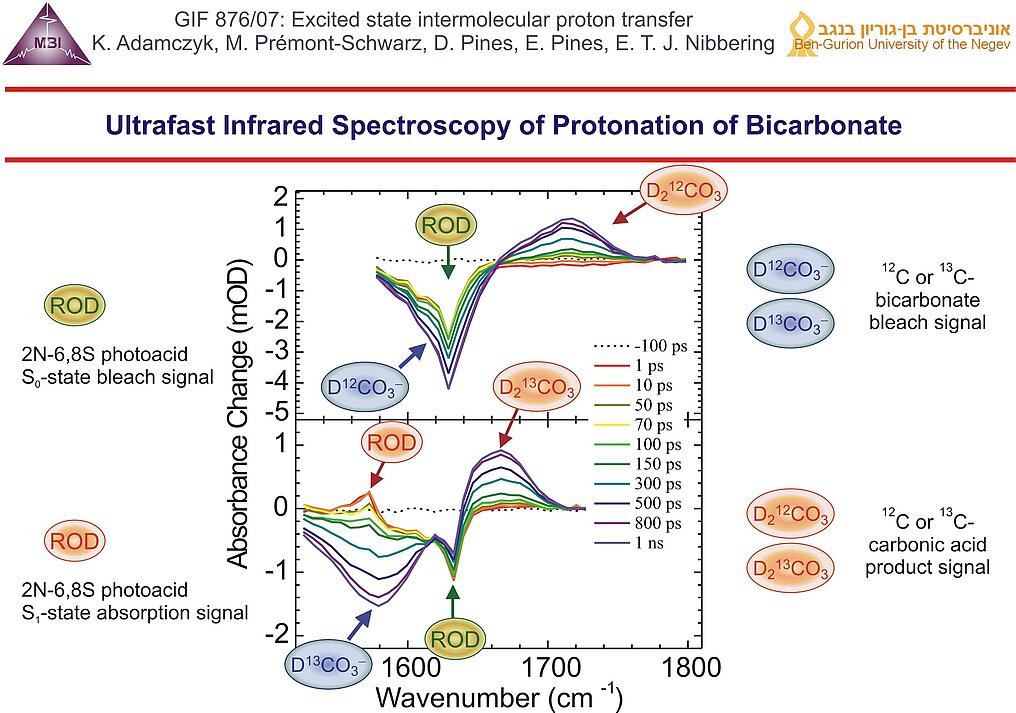
13. Formation dynamics of carbonic acid as function of base concentration: 0.25 M (red), 0.5 M (green) and 0.8 M (blue). At the highest base concentration the early time dynamics is dominated by encounter pair reactions, at longer pulse delay the dynamics is governed by diffusional motions of photoacid and base. The experimentally found reaction rate for the protonation of bicarbonate (red dot) is only consistent with previously obtained protonation rates of carboxylate bases CH3-xClxCOO- (blue triangles) and HCOO-(black square) when the real acidity for carbonic acid is used: pKa = 3.45 ± 0.15. In contrast the effective value for CO2/H2O cannot be used for the results obtained with the ultrafast protonation experiment.
14. The facts that carbonic acid has an acidity between that of chloroacetic acid and formic acid, and the carbonic acid is relatively long lived suggests strongly the possibility that carbonic acid plays a key role as an intact molecule in the CO2 household in living animals, in chemical weathering of rock formations, in the CO2 household of Earth and in CO2 sequestration schemes where water is present.