3.1 Dynamics of Condensed Phase Molecular Systems
Project coordinators: E. T. J. Nibbering , O. KornilovPhase 5 (2014-2017): Ultrafast dynamics of hydrated DNA and RNA, of phosphate ions and of hydrated protons
The people involved:
Fabian Dahms, Yingliang Liu, Tobias Tyborgski, Eva-Maria Brüning, Rene Costard, Biswajit Guchait, Torsten Siebert, Benjamin Fingerhut, Erik T. J. Nibbering, Thomas Elsaesser
National and international collaboration: Ehud Pines,†
†: Department of Chemistry, Ben Gurion University of the Negev, Beer-Sheva, 84105 Israel
Hydrogen bonding of hydrated biomolecular and biomimetic systems is investigated with ultrafast vibrational spectroscopy. Hydration of biomolecules is addressed on the femto- to picosecond time scale by probing vibrational marker modes sensitive to molecular motions, energy exchange, and structural fluctuations of the hydration shell. Using artificial DNA oligomers as a model system and implementing a technique which allows for femtosecond measurements at a controlled variable hydration level, femtosecond 2D infrared and pump-probe spectroscopy is applied to provide new insight into hydration processes. Experiments on DNA oligomers and base pairs in solution addresses vibrational dynamics within the complementary hydrogen bonding structural motifs of base pairing, giving detailed information on the coupling and relaxation behaviour of the N-H stretching oscillators in these base pairs. The vibrational dynamics of phosphate groups in the DNA backbone and their energy exchange with the water shell have been determined for the first time, demonstrating that the surrounding water layers represent the preferential heat sink for excess energy released in DNA relaxation processes. These findings are complemented and confirmed by measurements on water pools nano-confined in reverse micelle structures with phosphate head groups.
Phase 5 (2014-2017):
In the fifth period we targeted our research on hydration phenomena of DNA oligomers as well as of phosphate ions. A close collaboration with the junior theory group of Dr. Benjamin Fingerhut has been established. A new research line involves the vibrational dynamics of hydrated proton species.
5-1 Vibrational dynamics in hydrated DNA
5-2 Ultrafast dynamics of hydrated phosphate ions
5-3 Ultrafast dynamics of hydrated excess proton
Vibrational dynamics in hydrated DNA
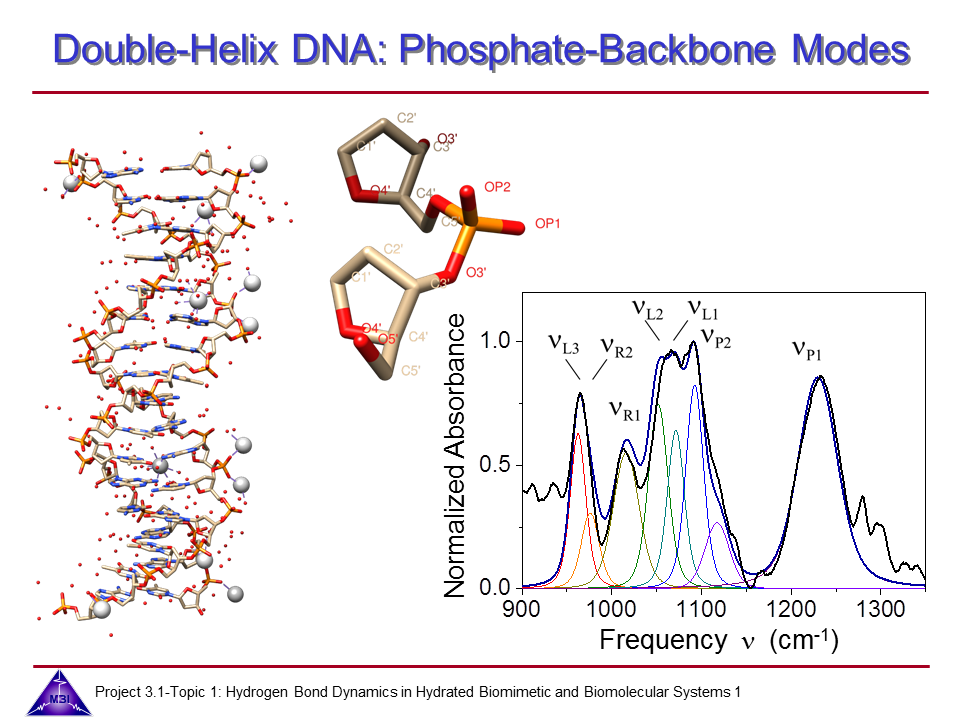
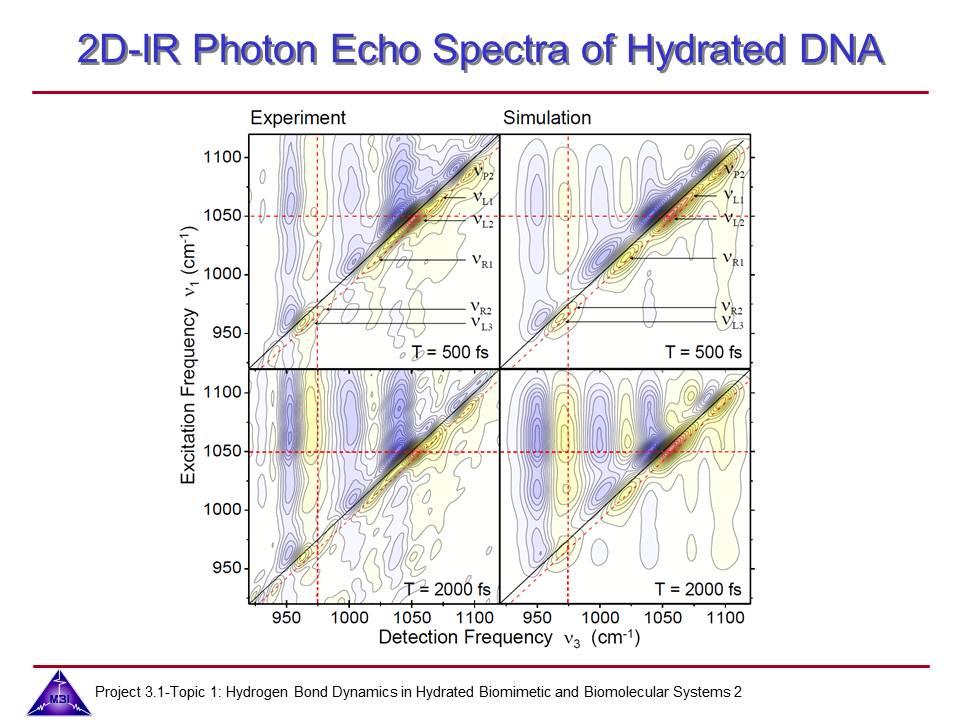
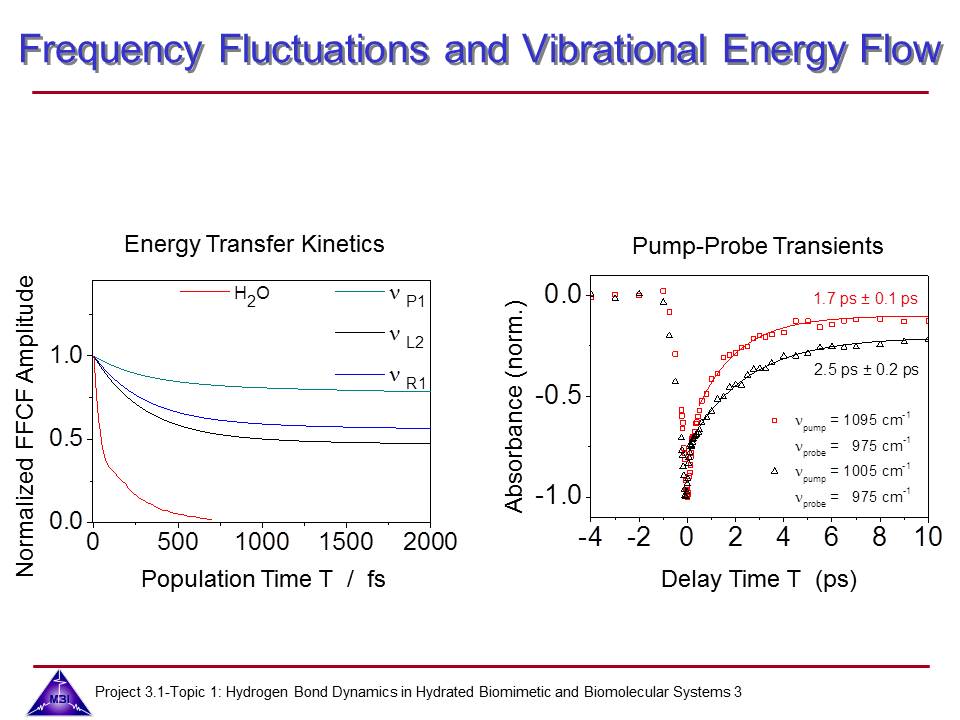
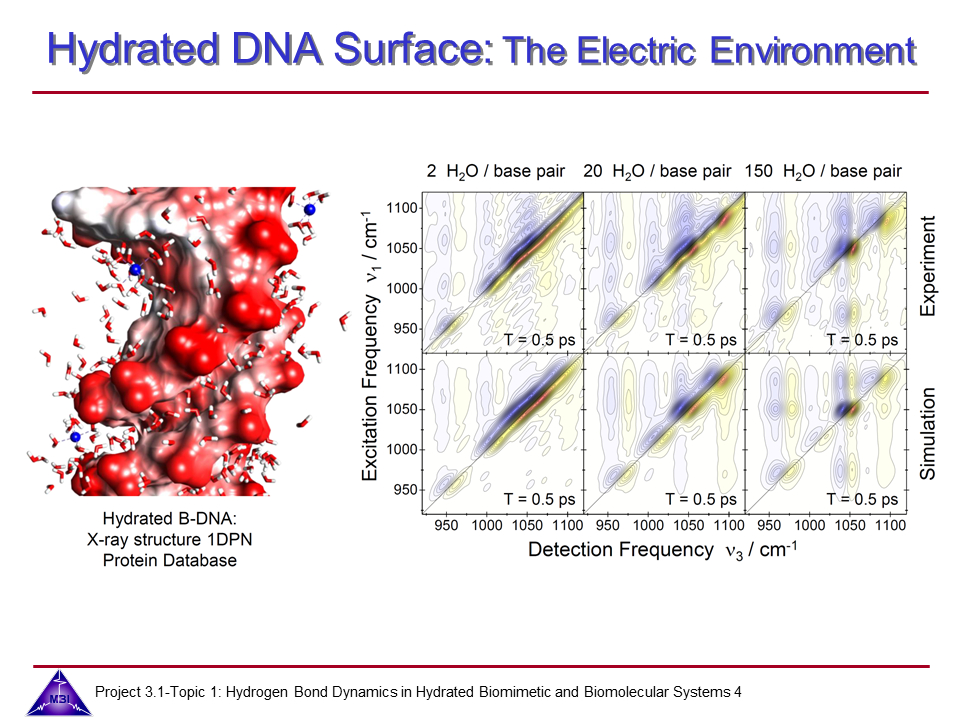
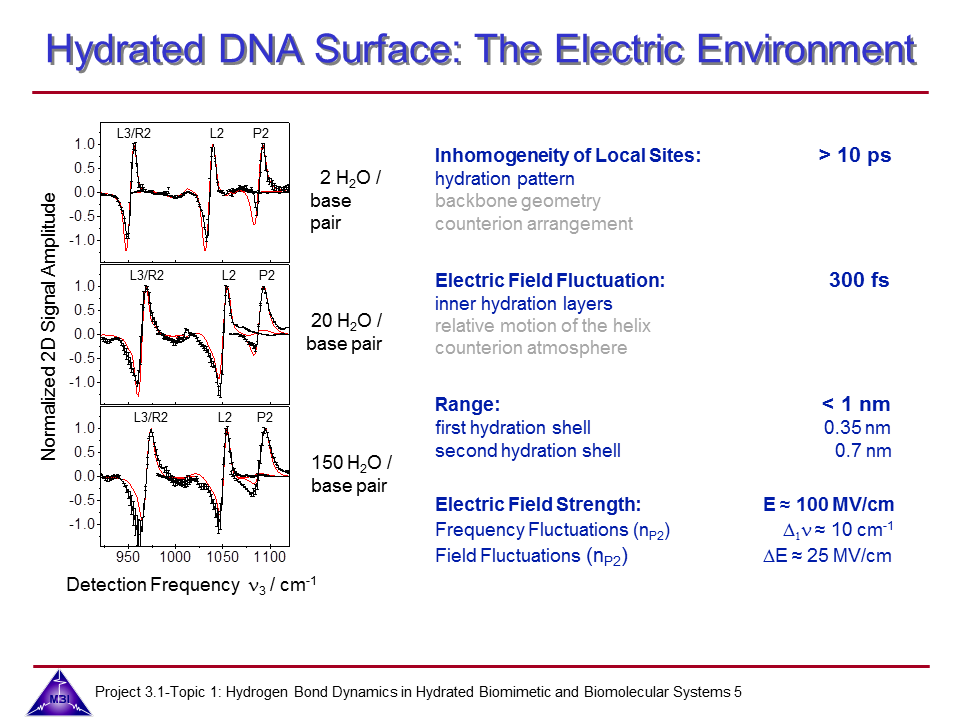
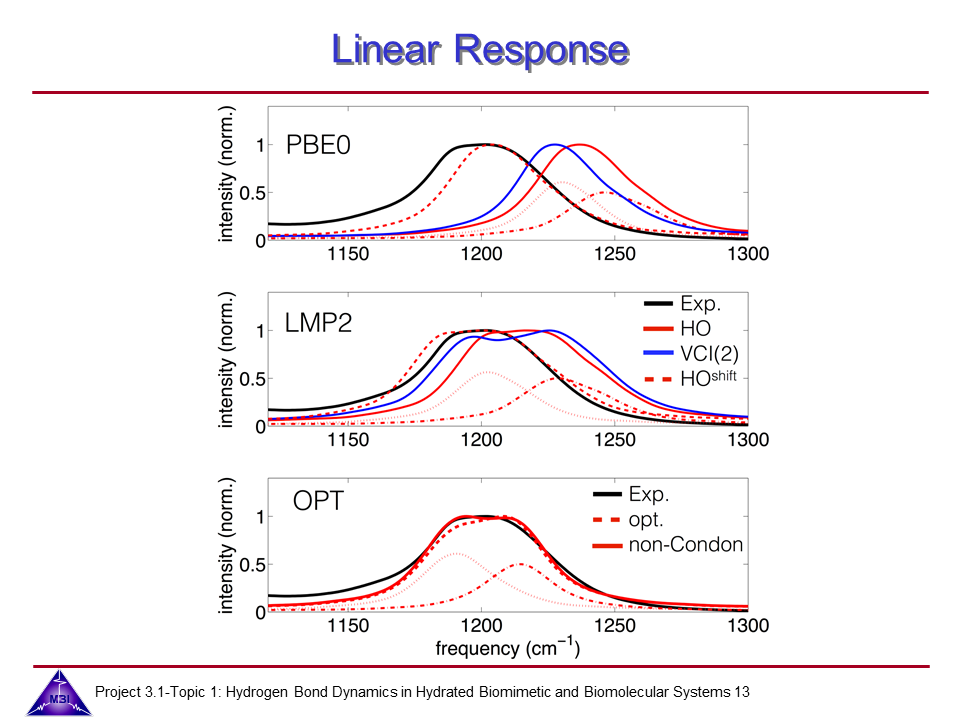
We continue our research on vibrational dynamics in hydrated DNA. Dynamics and couplings of phopshate and sugar backbone modes of Watson-Crick double helix DNA films (consisting of 23 oligomer adenine-thymine base pairs) have been explored with ultrafast IR pump-probe and 2D-IR spectroscopy.
Ultrafast dynamics of hydrated phosphate ions
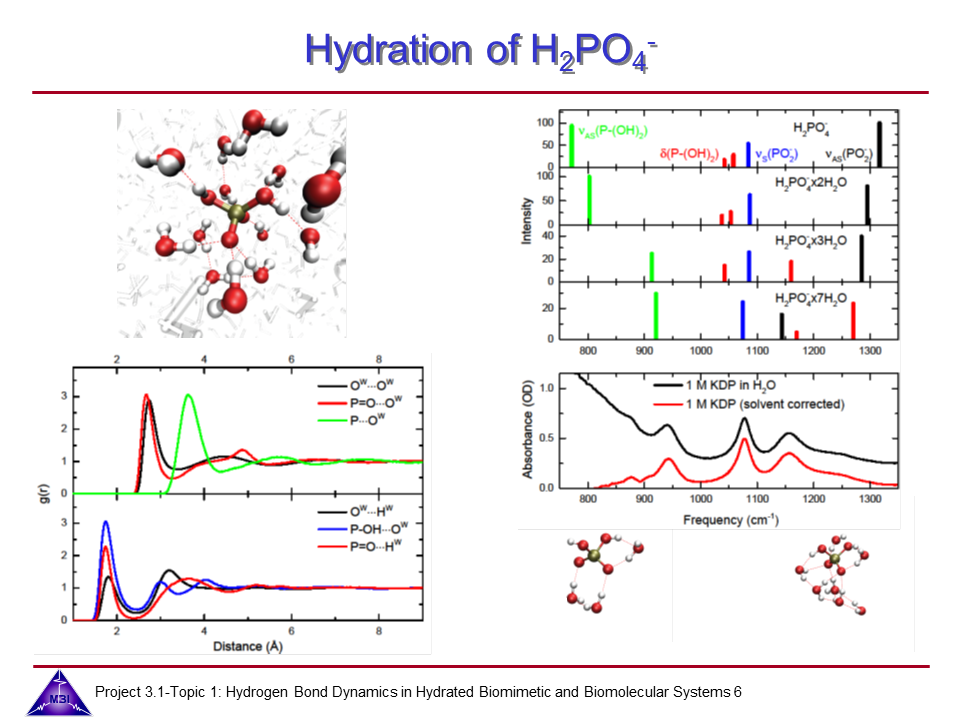
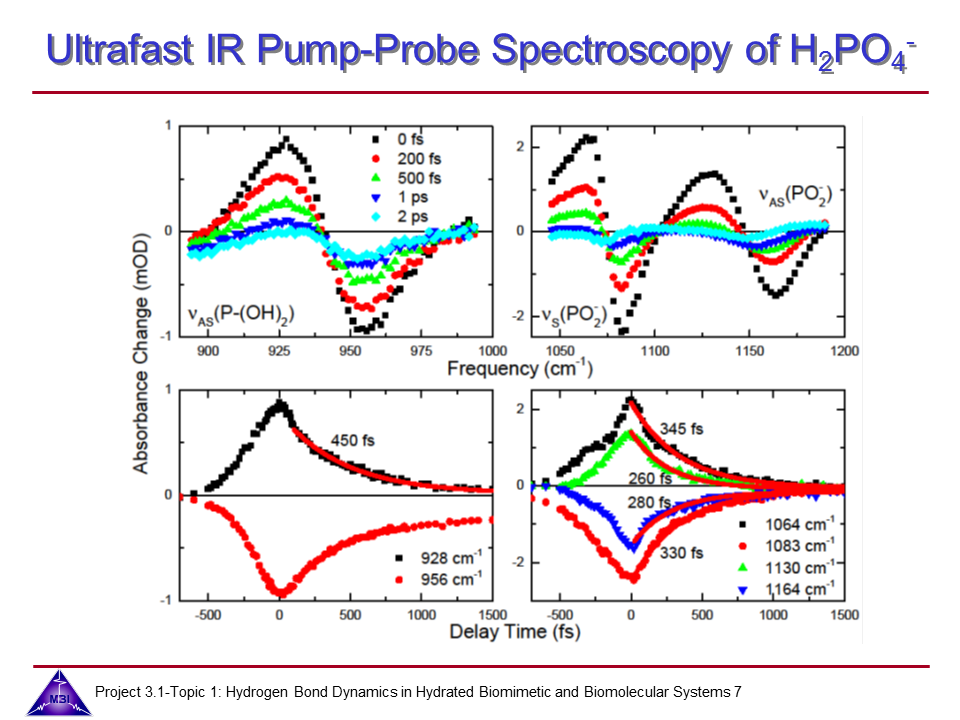
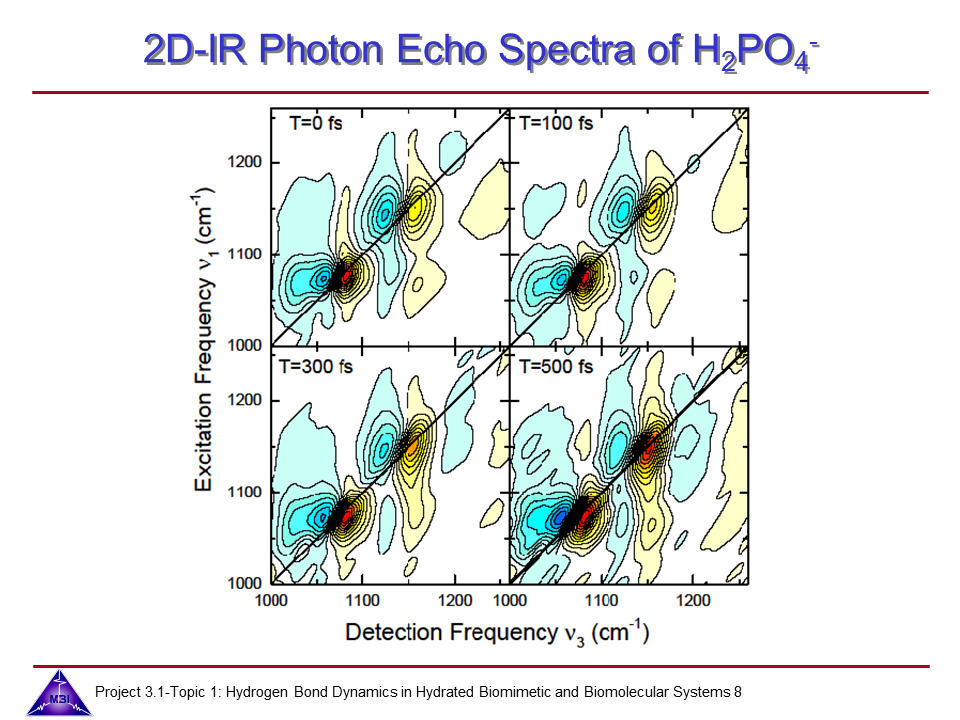
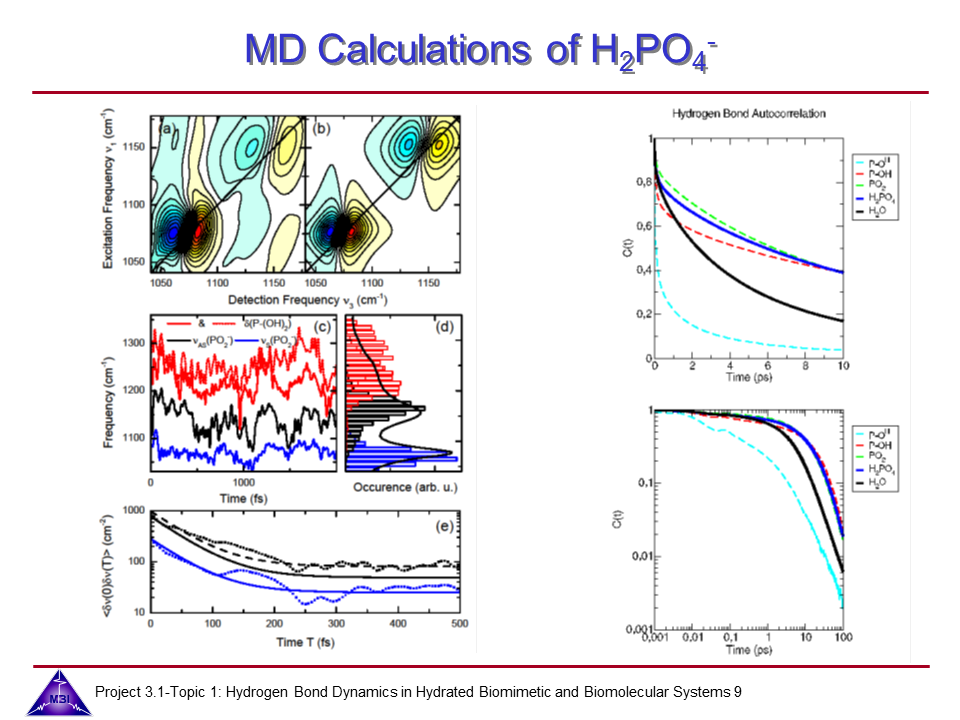
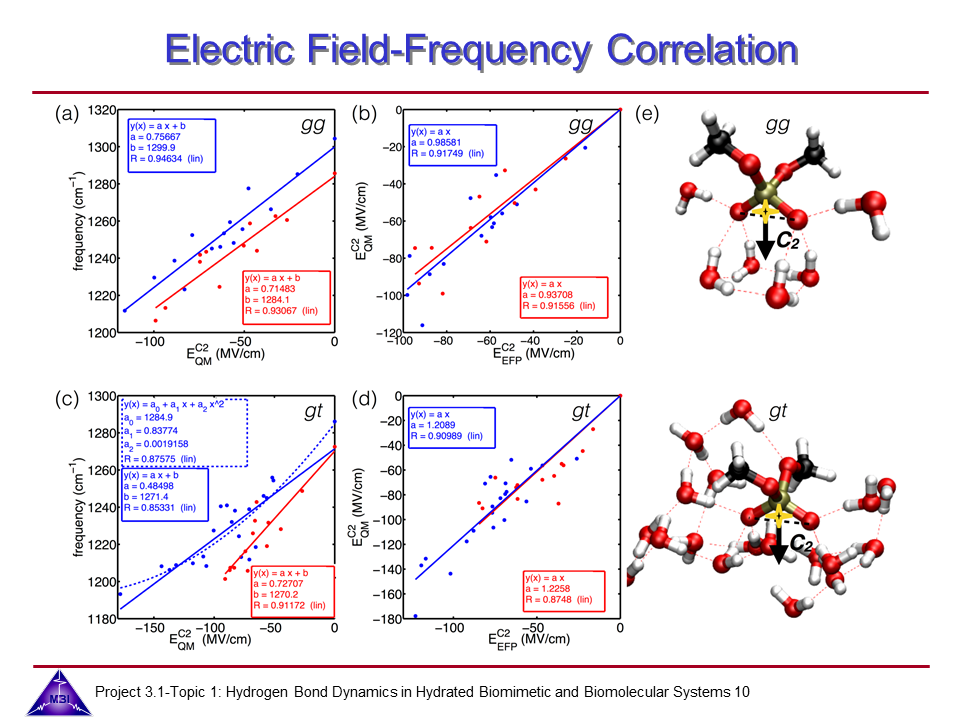
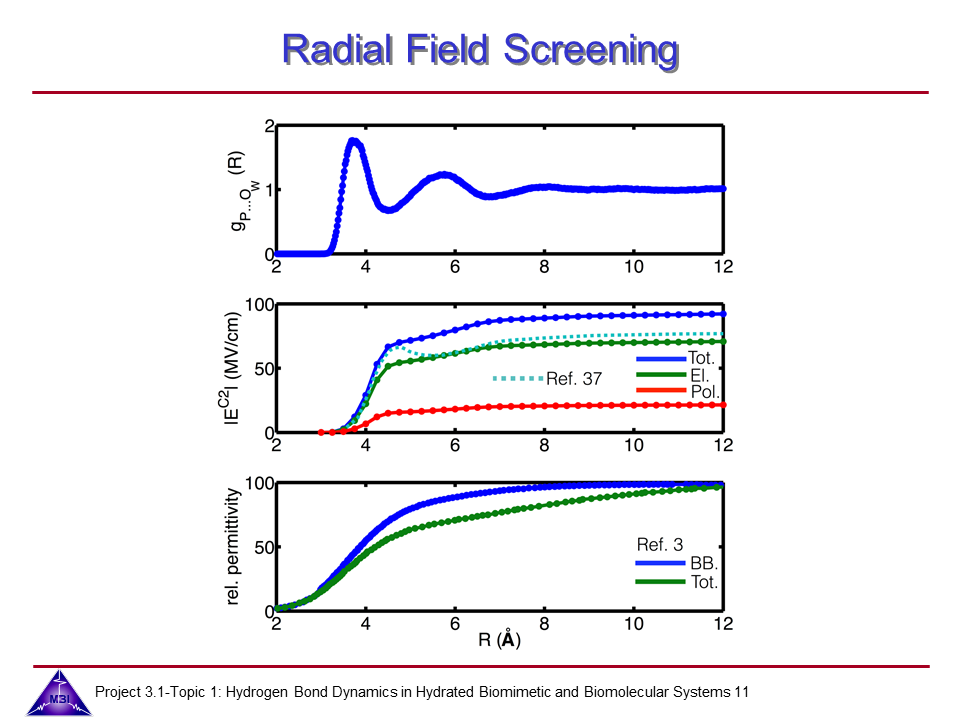
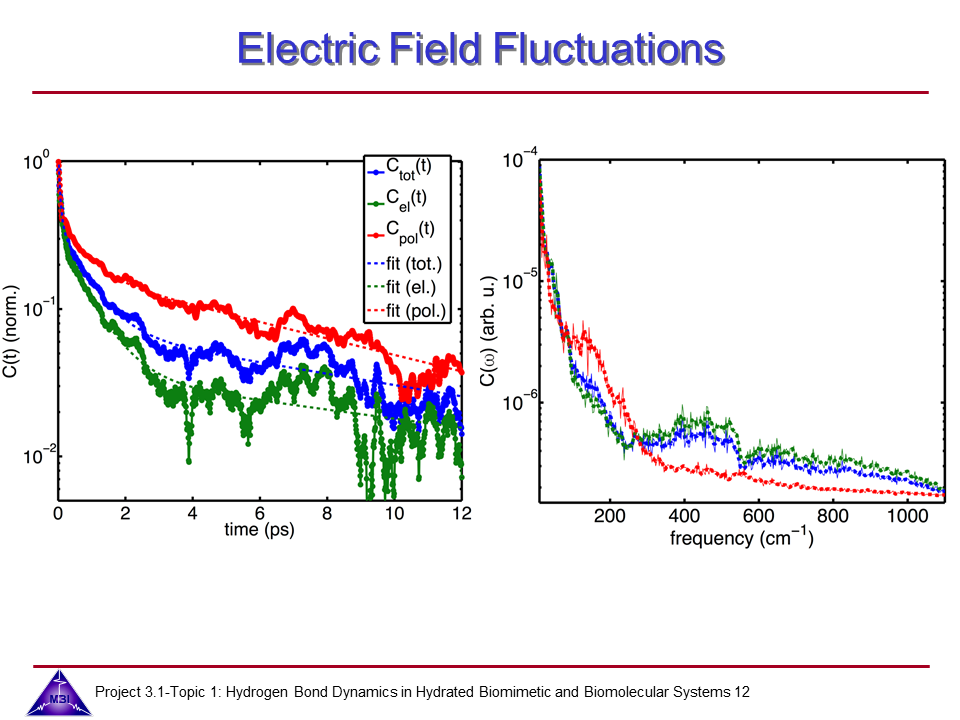
We investigate the vibrational dynamics of aqueous phosphate ions in a combined experimental and theoretical approach. The interaction of water molecules with ionic phosphate groups is of particular interest as phosphate groups are primary hydration sites in membranes and in biomolecules such as DNA and RNA.
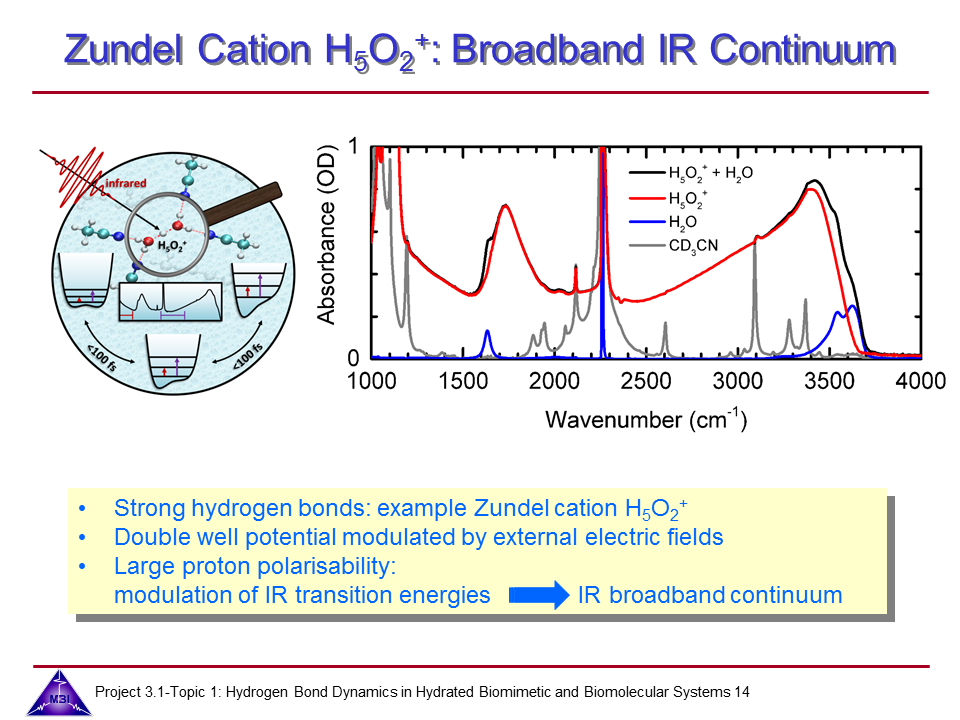
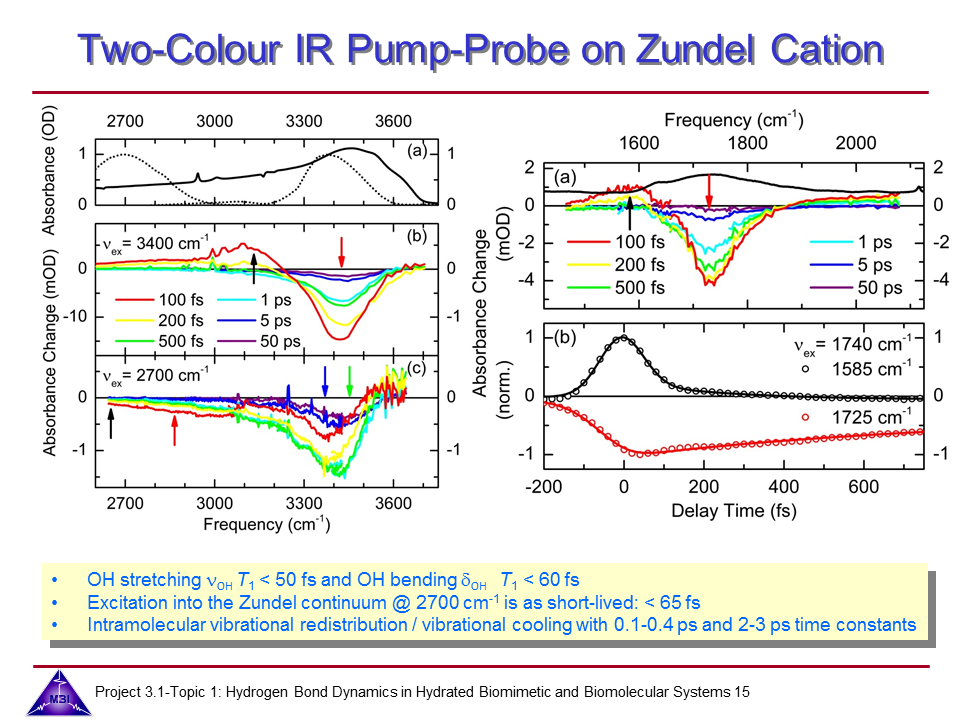
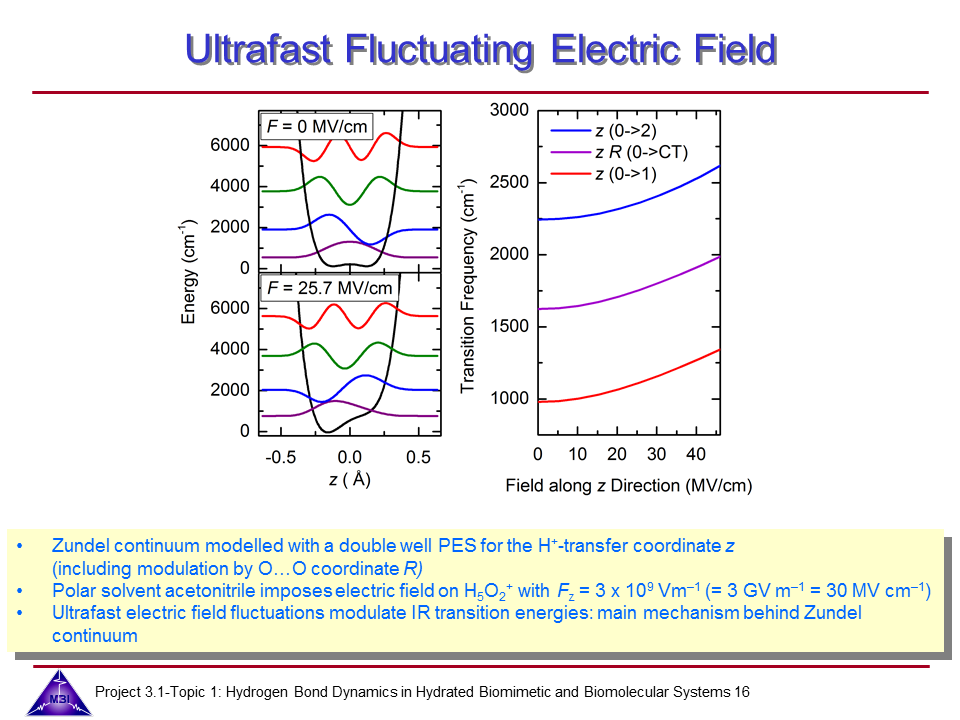
Ultrafast dynamics of hydrated excess proton
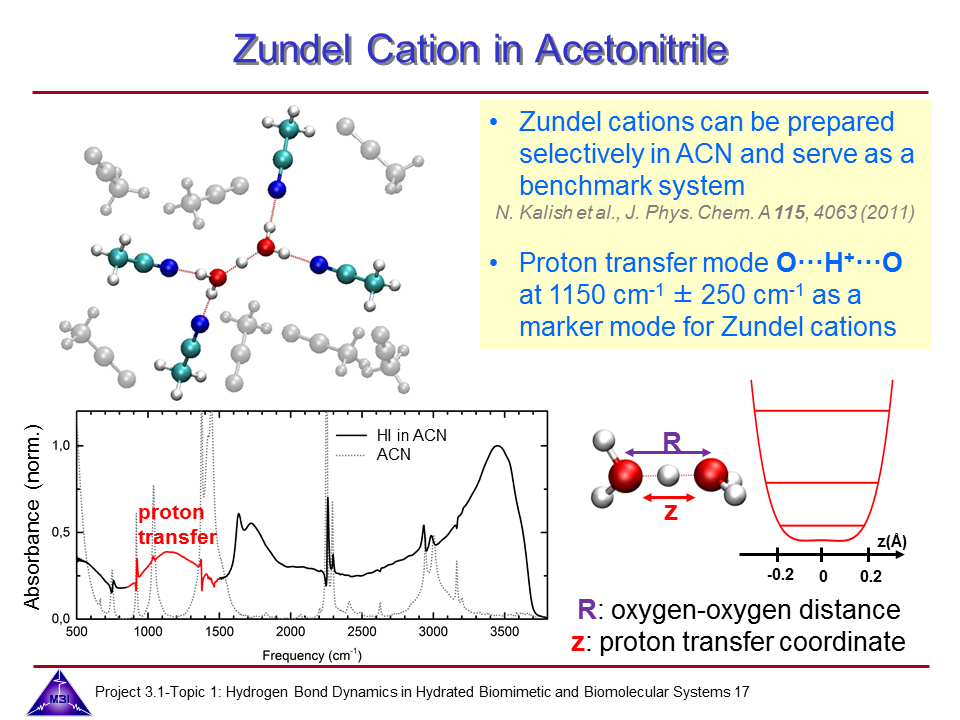
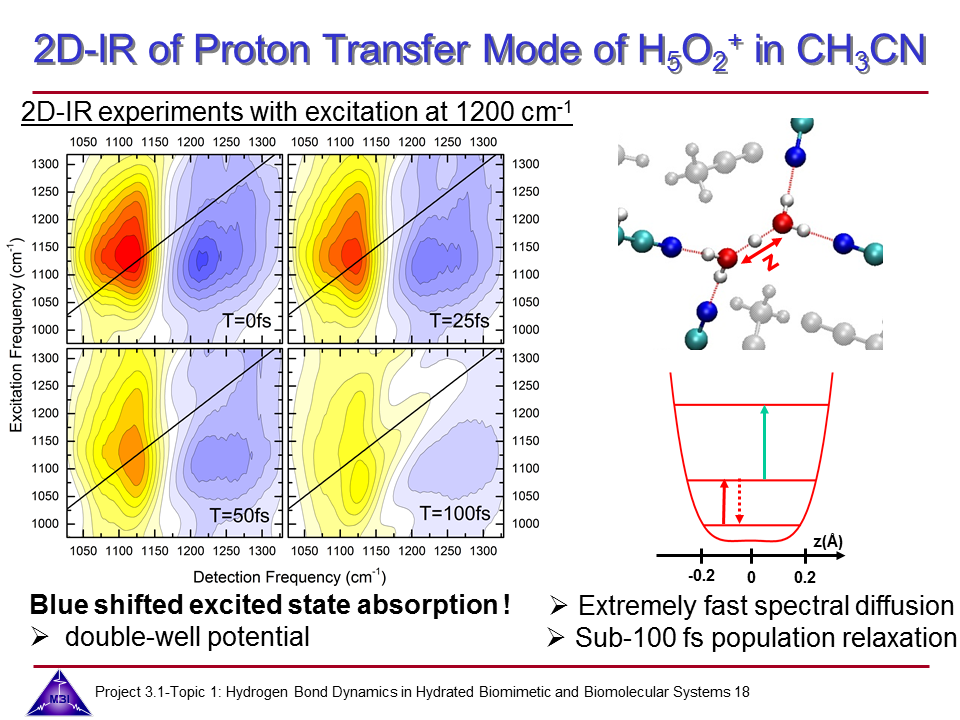
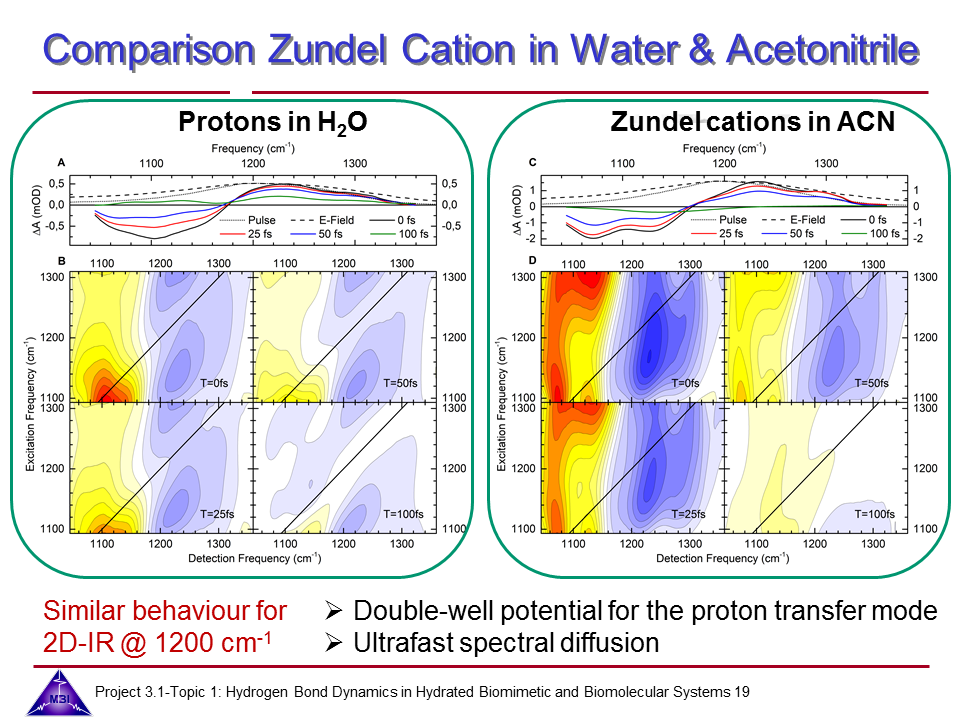
Hydrated protons are ubiquitous in aqueous acid-base reactions, in hydrogen fuel cells, and transmembrane proton channel proteins. Whereas numerous studies have focussed on isolated gas-phase hydrated proton-water clusters, we investigate the structure and dynamics of hydrated protons in the liquid phase. In our first studies we have focussed on the The Zundel cation H5O2+,