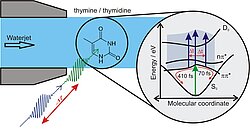
DNA stores our genetic code. Solar UV radiation is sufficiently high energy to break bonds in the DNA and thus cause DNA damage. However, even though DNA (eg in our skin cells) is exposed to intense UV rays from the sun daily, the DNA turns out to be astonishingly light-stable. It has long been known that this can be explained by mechanisms that highly efficiently transform electronic energy into other forms of energy, especially heat. Interfaces of the multidimensional potential energy surfaces, so-called conical intersections, play an important role between the electronically excited states and the electronic ground state. These conical penetrations are associated with structural changes in the molecules. The exact ways back to the electronic ground state are the subject of intensive research.
Although the DNA is a macromolecule with several billion atoms (in the case of human DNA), it can be divided into only a few different structural (and functional) elements: four DNA bases, a sugar residue and a phosphate group. The absorption of UV light takes place exclusively in the DNA bases. Therefore, it is a common research approach to first study only the reaction of the DNA bases to UV absorption.