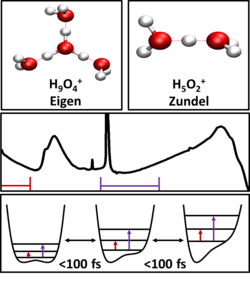
The proton (H+), the positively charged nucleus of a hydrogen atom, plays a fundamental role for many processes in nature. In liquid water, the transport of electrical charge is dominated by moving excess protons while proton motions across cell membranes are at the heart of cell respiration. In spite of this relevance, the molecular nature and dynamics of excess protons interacting with water molecules in their environment are not fully understood. Vibrational, in particular infrared spectroscopy has helped to identify limiting molecular structures of hydrated protons such as the Eigen and Zundel cations where the latter displays an extremely broad unstructured infrared absorption, a so-called "Zundel continuum" (Figure 1). In liquid water, such structures are unstable and expected to undergo rapid changes on a time scale of femto- to picoseconds (1 picosecond = 1 ps = 10-12 s). The mechanisms underlying the absorption continua have remained highly controversial.
Fluctuating liquid structure induces ultrabroad infrared absorption: The hydrated proton on ultrafast time scales
Fig. 1: Hydration of protons goes beyond the hydronium (H3O+) species typically mentioned in chemistry textbooks. Eigen and Zundel cations have been named after two leading German scientists Manfred Eigen and Georg Zundel who proposed these structures in the 1960s. The mid-infrared spectrum of the Zundel cation shows the marked contributions of OH stretching and bending vibrations, and the significant broadband Zundel continuum. This Zundel continuum is caused by the ultrafast fluctuating potential of the proton transfer coordinate that modulates fundamental, overtone and combination tone transitions.
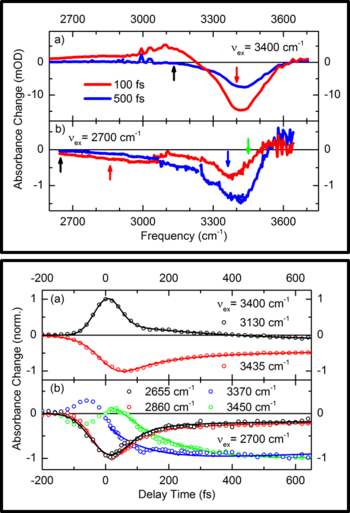
Researchers from the Max Born Institute for Nonlinear Optics and Short Pulse Spectroscopy in Berlin and the Ben Gurion University of the Negev in Beer-Sheva, Israel have now applied nonlinear infrared spectroscopy with femtosecond time resolution to elucidate the nature of the broadband continuum. For the particular model case H5O2+, the Zundel cation consisting of two water molecules held together by a proton (H2O...H+...OH2), they dynamically dissect the Zundel continuum from the regular OH stretching and bending vibrations of the two water molecules (Figure 2). As they report in Angewandte Chemie Int. Ed. (DOI: 10.1002/anie.201602523 ), a judicious choice of femtosecond vibrational excitation allows for isolating the transient continuum absorption. The different excitations show lifetimes below 60 fs, much shorter than the OH stretching and bending vibrations of neat water.
Fig. 2:Transient IR spectra showing the distinct response of the OH stretching mode and the Zundel continuum after femtosecond excitation
A theoretical analysis of the results demonstrates that the extreme broadening of the infrared absorption is caused by motions of the inner proton exerted by the strong, rapidly fluctuating electrical fields that originate from the surrounding polar solvent molecules. The energy of proton motions along the so-called proton transfer coordinate, the direction connecting the two water molecules in (H2O...H+...OH2), is strongly modulated by these external fields, resulting in a concomitant modulation of vibrational transition energies. On a time scale faster than 100 fs, the system explores a broad range of transition energies. Together with vibrational overtones, combination tones and modes changing the distance between the two water molecules the field modulated transitions lead to the observed extreme broadening of the infrared absorption. Due to the extremely fast structural fluctuations, particular H+ arrangements are washed out very rapidly, i.e., the system has an extremely short-lived structural memory.
This new view at the Zundel cation clearly goes beyond the many studies of gas phase cluster work on hydrated protons, where due to the low temperature conditions, the Zundel continuum is not observed. The results are of relevance for many dynamic aspects of hydrated protons, be it for proton transport in water by the infamous von Grotthuss mechanism, in hydrogen fuel cells, or biological systems functioning with proton translocation mechanisms.
Search publications of MBI
Publications since 2025