Interatomic Coulomb decay (known as ICD) describes the relaxation of an excited atom by transferring the excess energy to an adjacent atom that is ionized. This effect has attracted considerable attention in recent years as it could be a source of radiation damage in biological systems. At the same time it was proposed to use ICD for novel cancer therapies. So far, ICD has been observed after clusters of high-energy photons have been ionized or excited in the extreme ultraviolet (XUV) or X-ray region. In contrast, ICD was not expected to be triggered by low-energy photons in the NIR range.
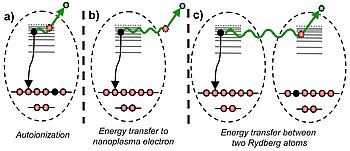
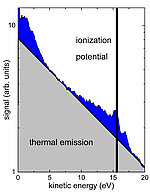
The ionization of a cluster with an intense NIR laser pulse introduces highly complex dynamics. A so-called nanoplasm is formed, consisting of a large number of electrons and ions interacting with each other. It has been observed that the recombination of electrons and ions leads to the generation of Rydberg atoms and ions, which can decay by fluorescence. However, in a strongly ionized cluster, Rydberg atoms may also relax by correlated electronic decay (CED), similar to the ICD, i. without the emission of radiation. CED means that an electron can relax from a Rydberg state to the ground state and transfer the excess energy to a second electron that is either in the same atom, in the nanoplasm, or in a Rydberg state of a nearby atom (see Figure 1). , With the help of this extra energy, the second electron can escape the cluster. "Although you can basically expect CED in nanoplasmas, the effect was neither experimentally observed nor predicted by theoretical models," explains Dr. Bernd Schütte from the Max Born Institute. "The big challenge in the experiment was to find suitable conditions that allow us to directly observe correlated electronic decay."