3.1 Dynamics of Condensed Phase Molecular Systems
Project coordinators: E. T. J. Nibbering , O. KornilovPhase 3 (2018-...): Water-Mediated Acid-Base Reactions

The people involved:
Maria Ekimova, Carlo Kleine, Jan Ludwig, Marius-Andrei Codescu, Oleg Kornilov,Sebastian Eckert, Marc-Oliver Winghart, Peng Han, Debkumar Rana, Zhuang-Yan Zhang, Erik T. J. Nibbering
National and international collaboration: Felix Hoffmann,‡ Gül Bekçioğlu,‡ Thomas Kunze,‡ Moritz Weiß,‡ Martin Brehm,‡ Wilson Quevedo,+ Markus Kubin,+ Rolf Mitzner,+ Mattis Fondell,+ Miguel Ochmann,o Jessica Harich,o Thomas E. Agrenius,* Ambar Banerjee,* Eve Kozari,† Dina Pines,† Subhajyoti Chaudhuri,#Atanu Acharya,# Uriel Morzan,# Micheline Soley,# Pablo E. Videla,#Daniel Sebastiani,‡ Victor S. Batista,# Nils Huse,o Philippe Wernet+.‖, M. Odelius,* Ehud Pines†
‡: Institut für Chemie, Martin-Luther-Universität Halle-Wittenberg, Von-Danckelmann-Platz 4, 06120 Halle (Saale), Germany
+: Ultrafast X-Ray Science Group, Helmholtz-Zentrum Berlin, BESSY II, Albert-Einstein-Str. 15, D-12489 Berlin, Germany
o: Institute for Nanostructure and Solid State Physics, Center for Free-Electron Laser Science, University of Hamburg, Germany
‖: Department of Physics, University of Uppsala, Uppsala, Sweden
*: Department of Physics, Stockholm Universitet, Stockholm, Sweden
†: Department of Chemistry, Ben Gurion University of the Negev, Beer-Sheva, 84105 Israel
#: Department of Chemistry, Yale University, P.O. Box 208107, New Haven, Connecticut 06520-8107, United States of America
Photoacids are used to investigate the elementary steps in water-mediated proton transfer reactions. IR and soft-x-ray spectroscopy are used as local probing tools on transient vibrational and electronic structure. Femtosecond UV-pump/IR-probe spectroscopy is used to follow the response of the photoacids, hydration shell water and bases during different stages of the proton transport. Soft-x-ray spectroscopy using flatjet technology is used to explore hydrogen bonding interactions of photoacids and bases, and will be further developped as technique probing hydrogen bonding interactions on ultrafast time scales. Proton transport along hydrogen-bonded solvent bridges connecting acid and base sites of bifunctional photoacids has been studied in a combined experimental and theoretical approach since 2015. This research is in part funded by a DFG Normalverfahren NI 492/13-2 / SE 1008/11-1 jointly with D. Sebastiani (2015-2017 and 2021-...). Since 2018 third party funding comes from an ERC Advanced Grant (XRayProton, Nibbering, 2018-2023).
Highlight I: One slide summary.
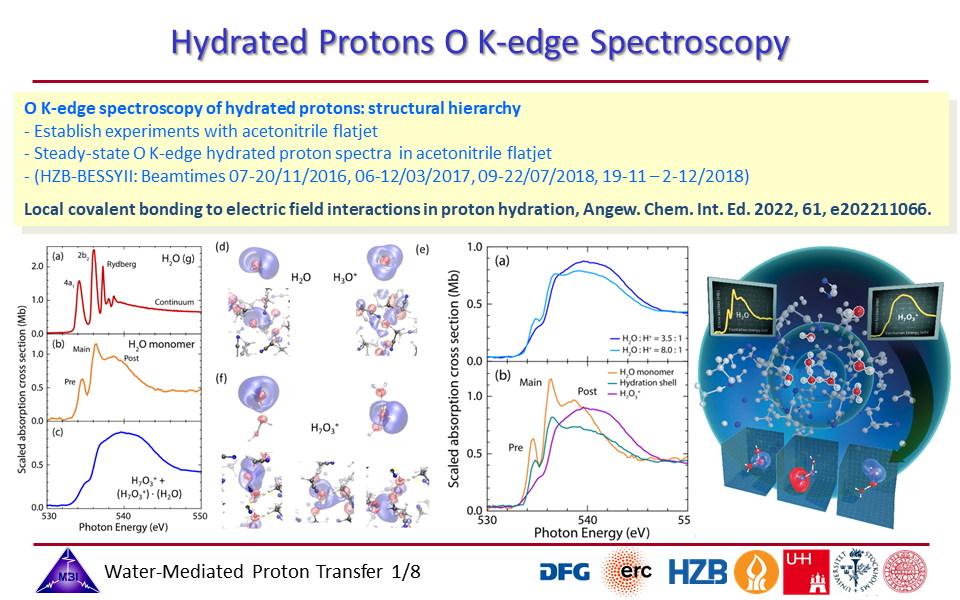
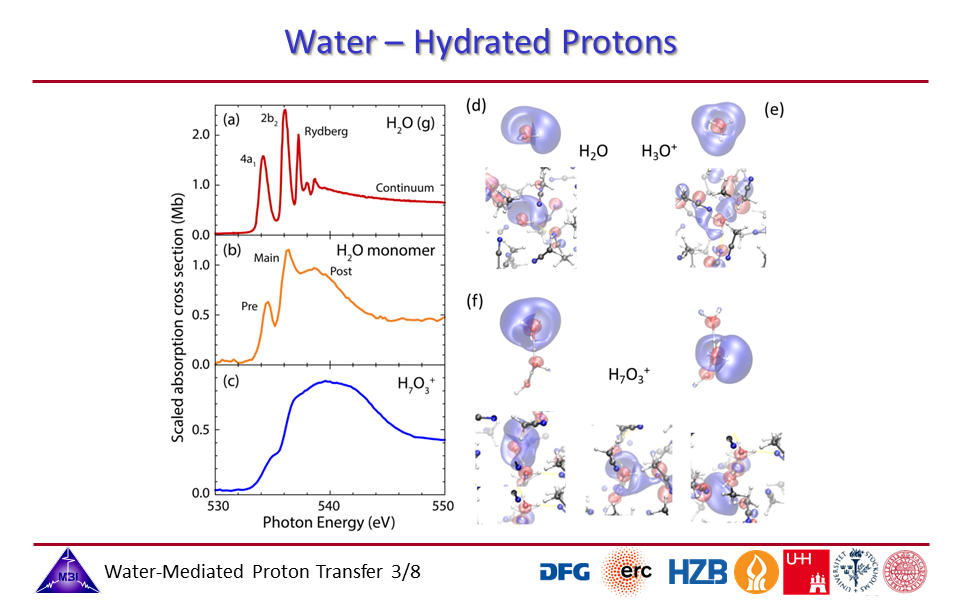
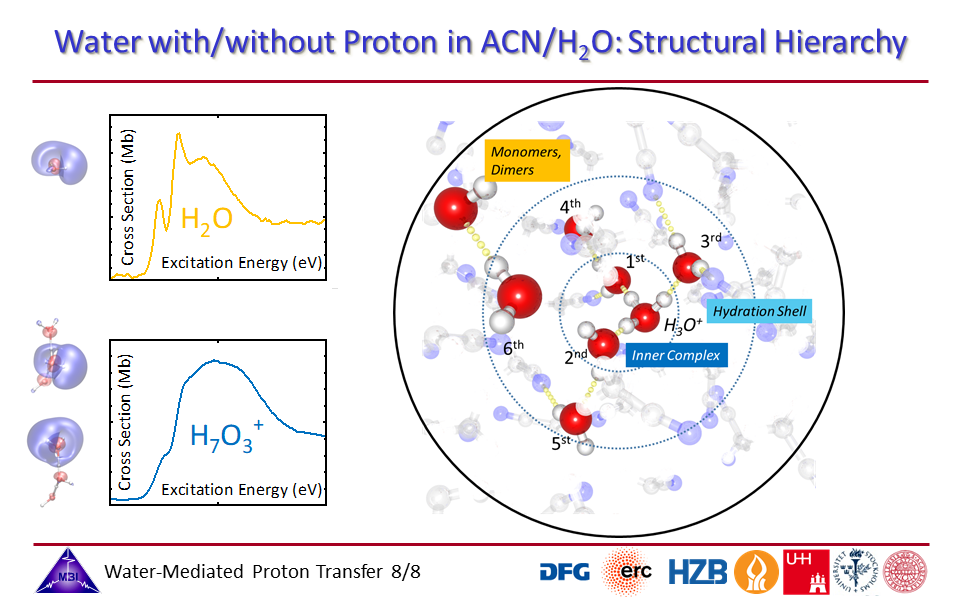
1.1. Oxygen K-edge spectroscopy of hydrated proton complexes in solution provides compelling evidence of the pronounced impact on the electronic structure of the water molecules participating in the hydration, with a hierarchy in coupling strengths of the proton with profoundly strong orbital interactions for the inner three water molecules, and Coulombic field effects of the proton positive charge with the first hydration shell water molecules.
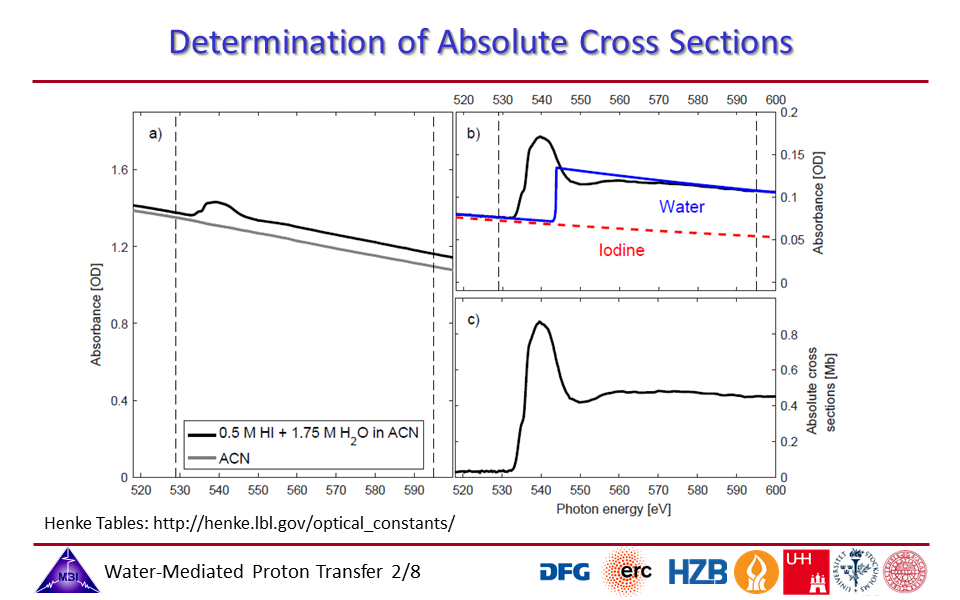
1.2. Determination of the absolute cross sections of the hydrated proton complex in acetonitrile,
1.3. Oxygen K-edge spectra of isolated water in the gas phase, water monomer in acetonitrile, and the hydrated proton complex in acetonitrile.
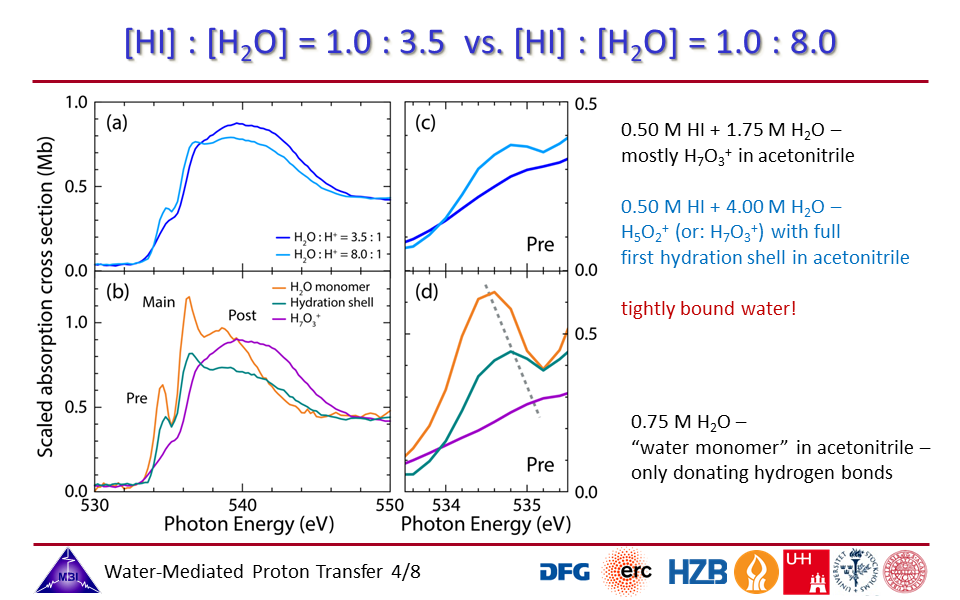
1.4. [HI]:[H2O] = 3.5 vs. [HI]:[H2O] = 8.0 mixing ratios: contributions of inner protonated water complex modified by strong orbital interactions and of hydration shell water molecules.
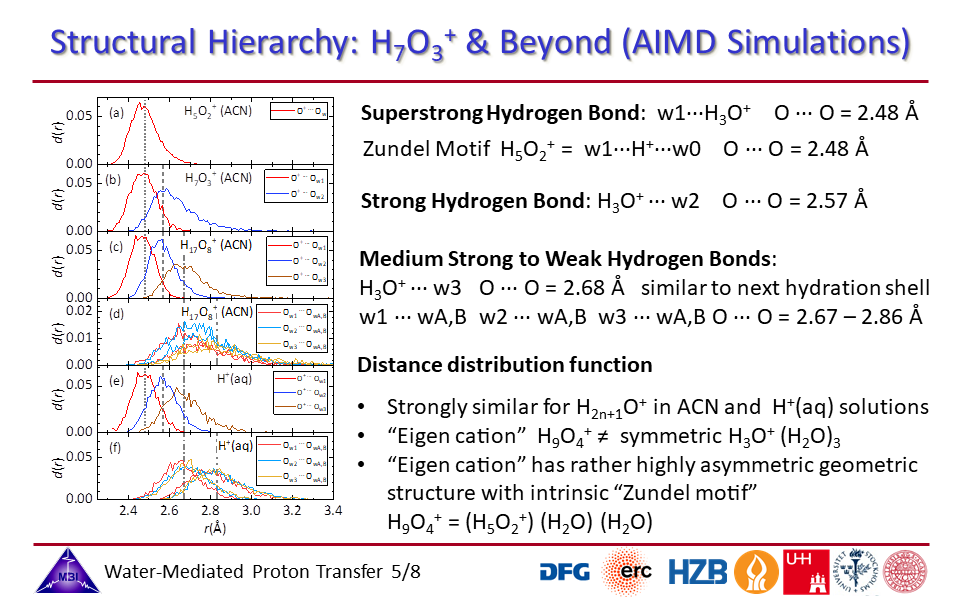
1.5. Ab initio molecular dynamics simulations of the hydrated proton complex in acetonitrile and in aqueous solution show similar distributions of supestrong, strong, medium strong and weak hydrogen bonded configurations.
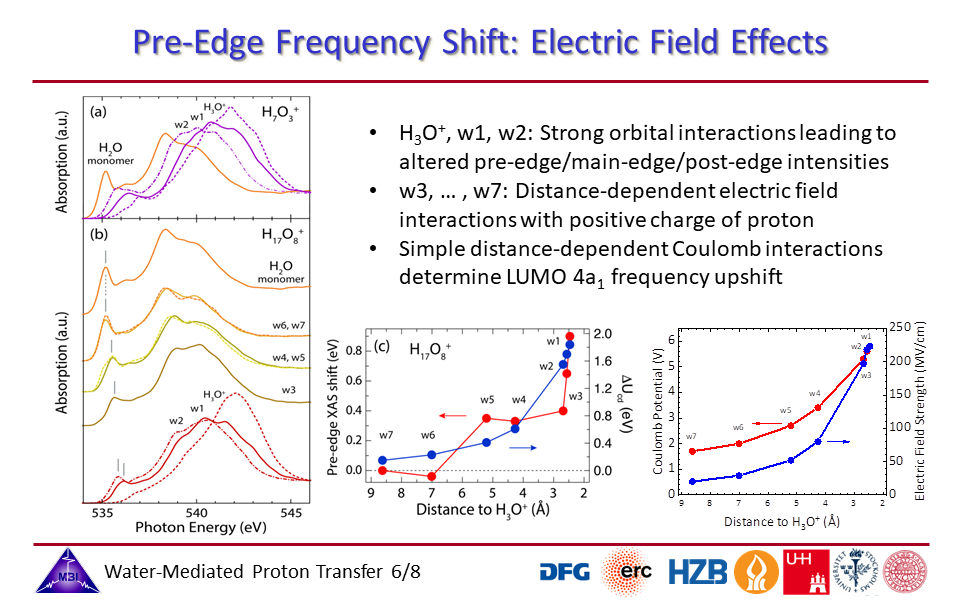
1.6. Electric field effects by the positive charge of the proton on the hydration shell water molecules.
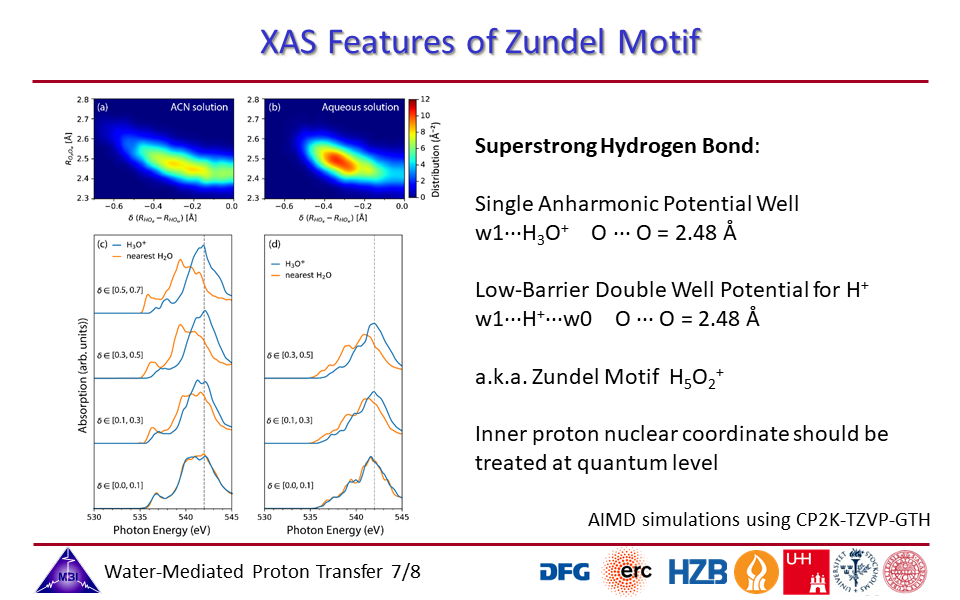
1.7. Zundel motif of innermost superstrong hydrogen bonded water molecules.
1.8. Structural hierarchy of hydrated proton complexes.
Highlight II: One slide summary.
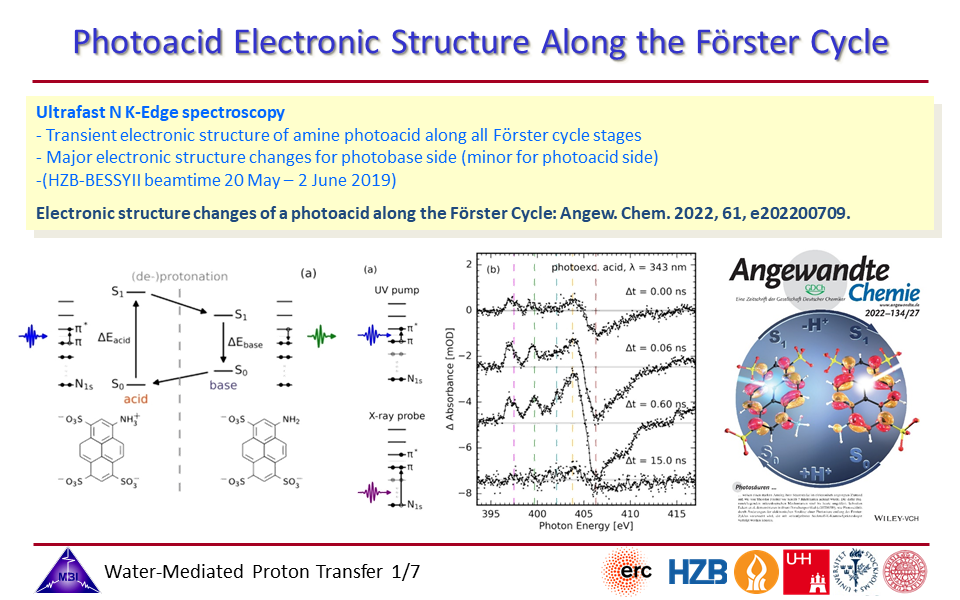
2.1. Electronic structural changes along the Förster cycle of an aromatic amine photoacid are monitored with time-resolved nitrogen K-edge spectroscopy, providing key insight into the factors responsible for the photoacidity properties.
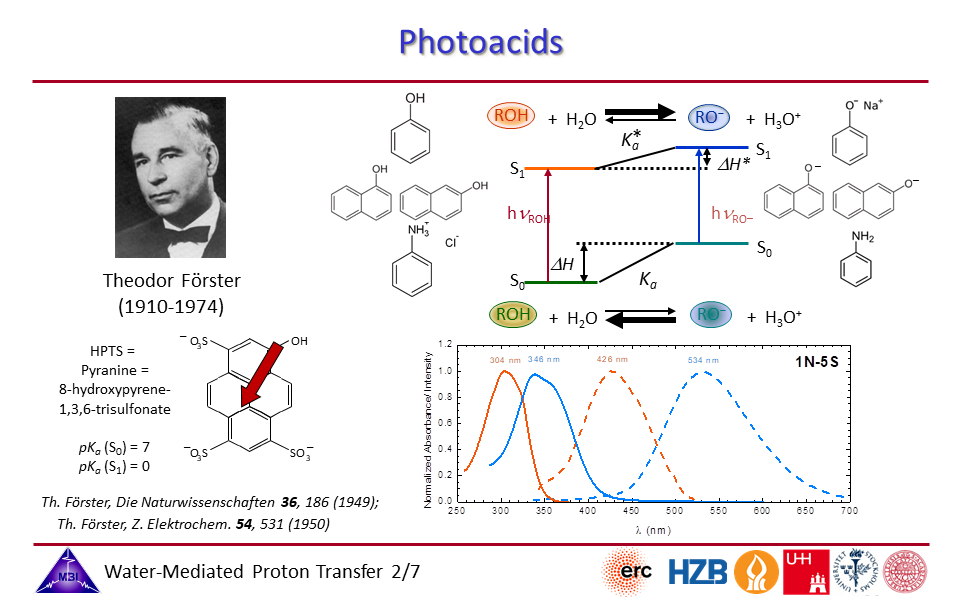
2.2. Photoacids typically can be characterized by the Förster cycle, connecting absorption/emission transitions with acid strengths.
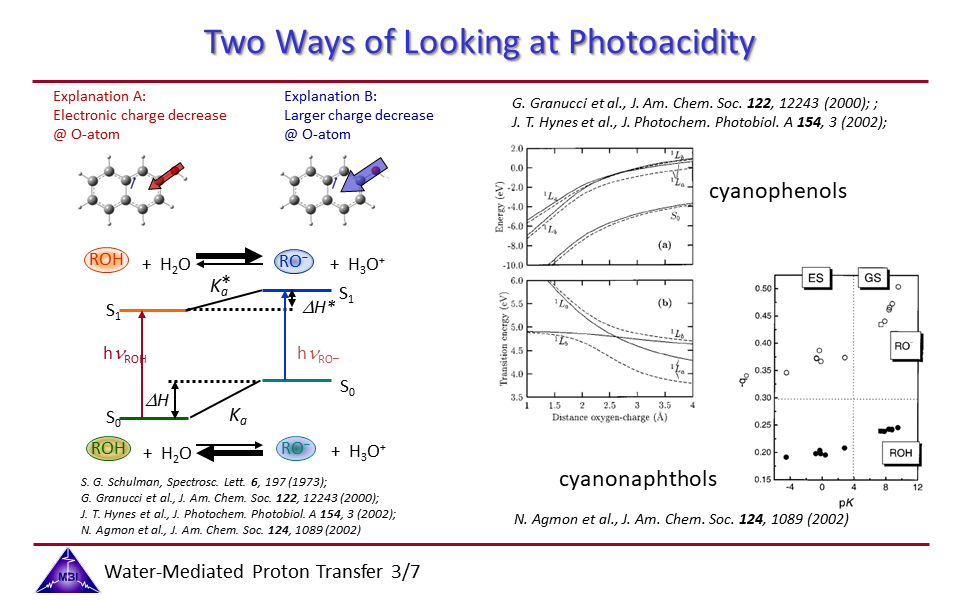
2.3. Two ways to desribe the mechanism of the photoacid phenomenon.
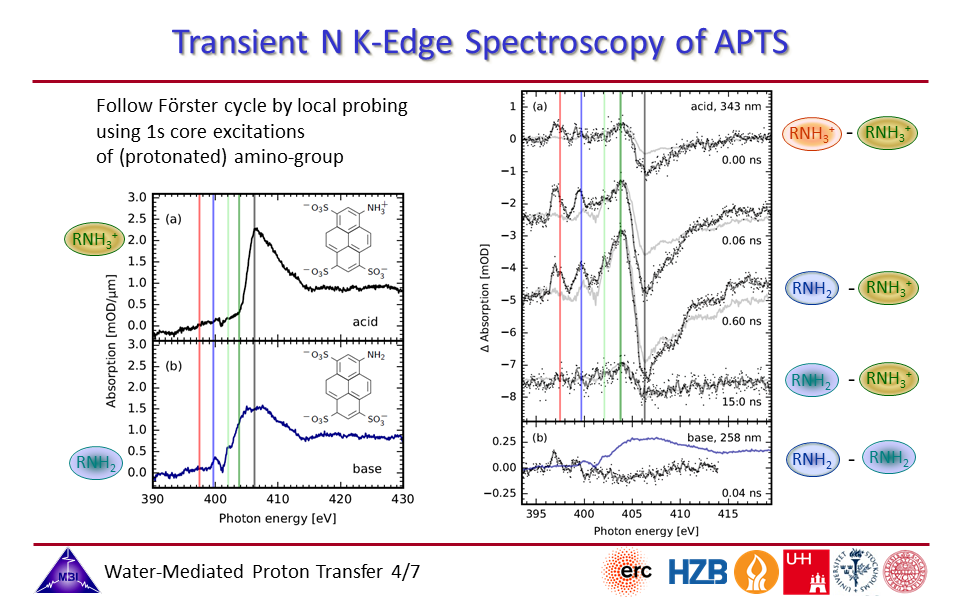
2.4. Steady-state and time-resolved nitrogen K-edge spectroscopy of 1-aminopyrene-3,6,8-trisulfonate (APTS).
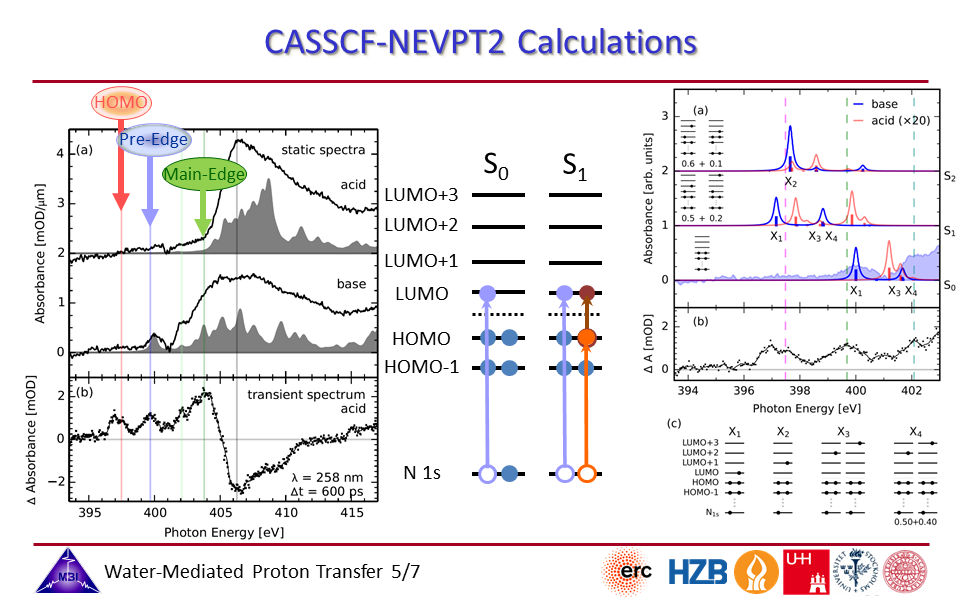
2.5. CASSCCF-NEVPT2 calculations of APTS spectra.
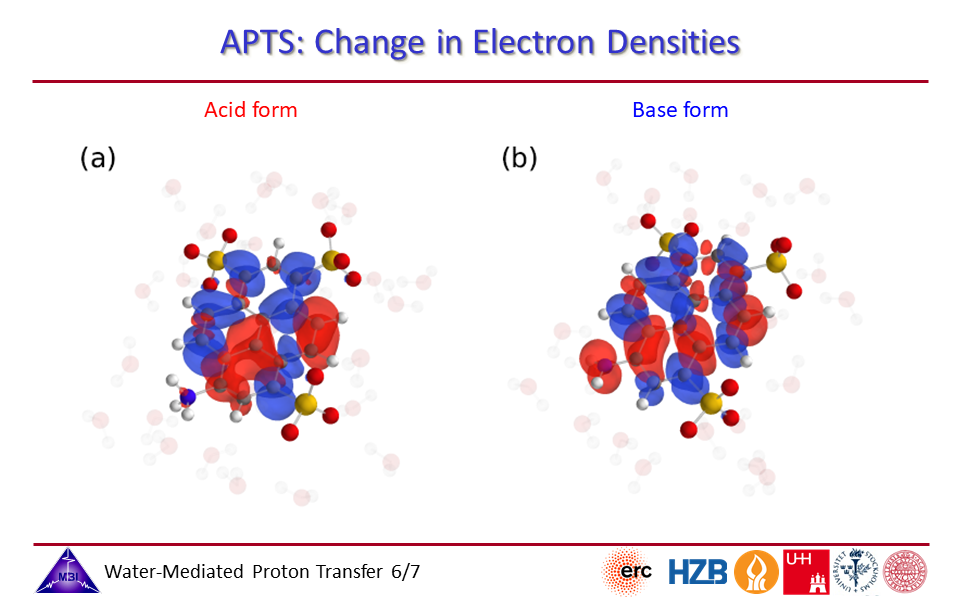
2.6. Change in electron densities for photoacid and for conjugate photobase side of APTS.
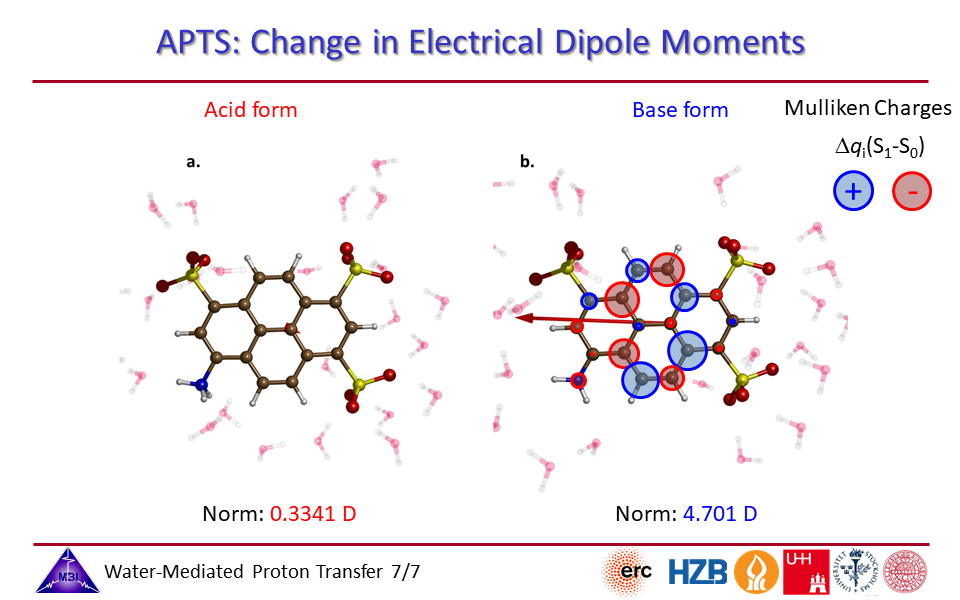
2.7. Change in electrical dipole moments for photoacid and for conjugate photobase side of APTS.
Highlight III: One slide summary.
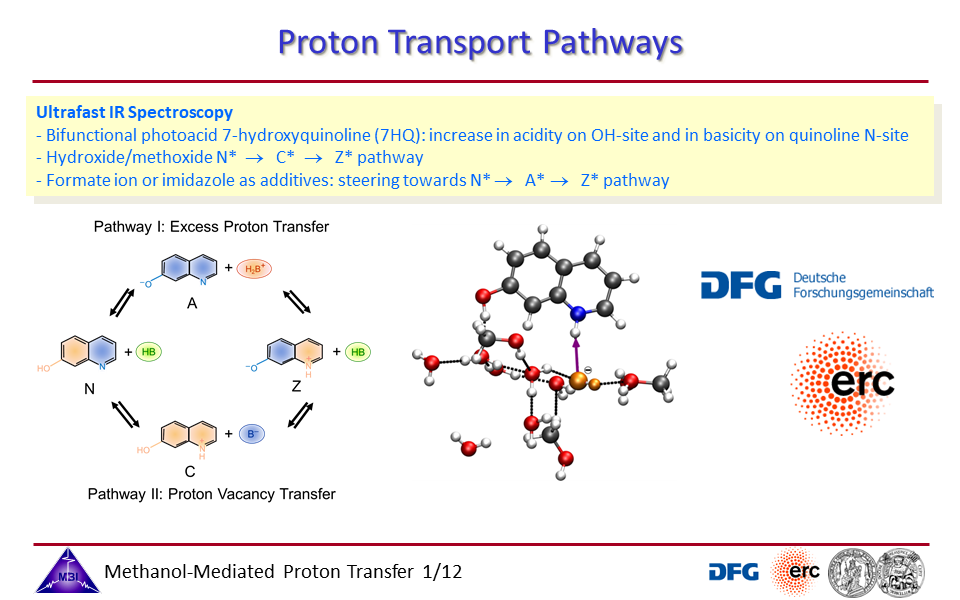
3.1. Scientists of the Max Born Institute of Nonlinear Optics and Short Pulse Spectroscopy (MBI) and the Martin-Luther-University Halle-Wittenberg (MLU) shows that proton vacancies in the form of hydroxide/methoxide ions are as relevant for proton transfer between acids and bases as hydrated excess protons (H3O+, H5O2+), thus pointing for a clear demand for refinement of the microscopic picture for aqueous proton transport – in solution as well as in hydrogen fuel cells or transmembrane proteins – away from currently often assumed dominant role of hydrated excess protons.
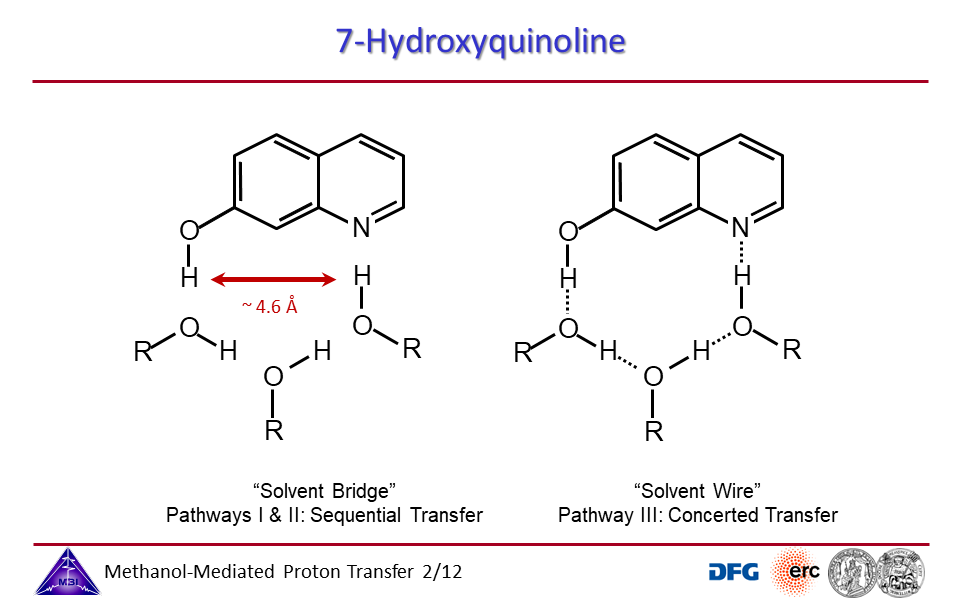
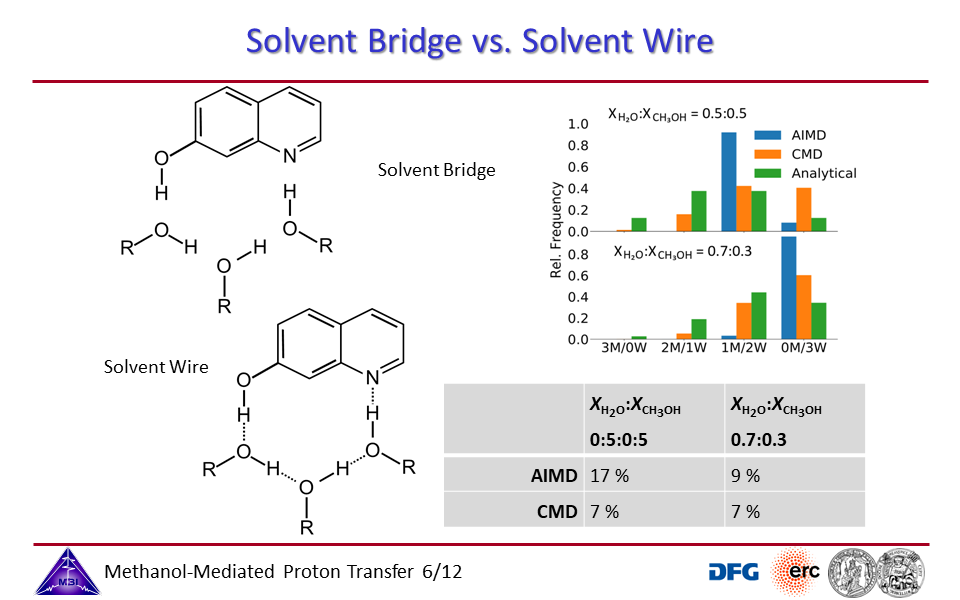
3.2. Proton exchange may occur through solvent bridges, with continously changing hydrogen bond interactions, or through a solvent wire, with a rigid solvent molecule arrangement.
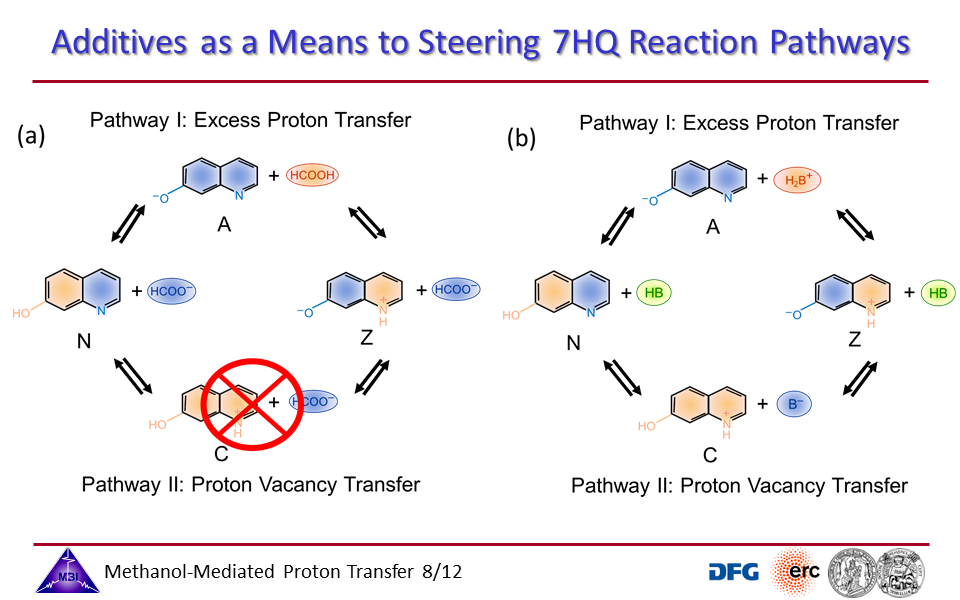
3.3. Proton transport through proton excess or through proton hole transfer.
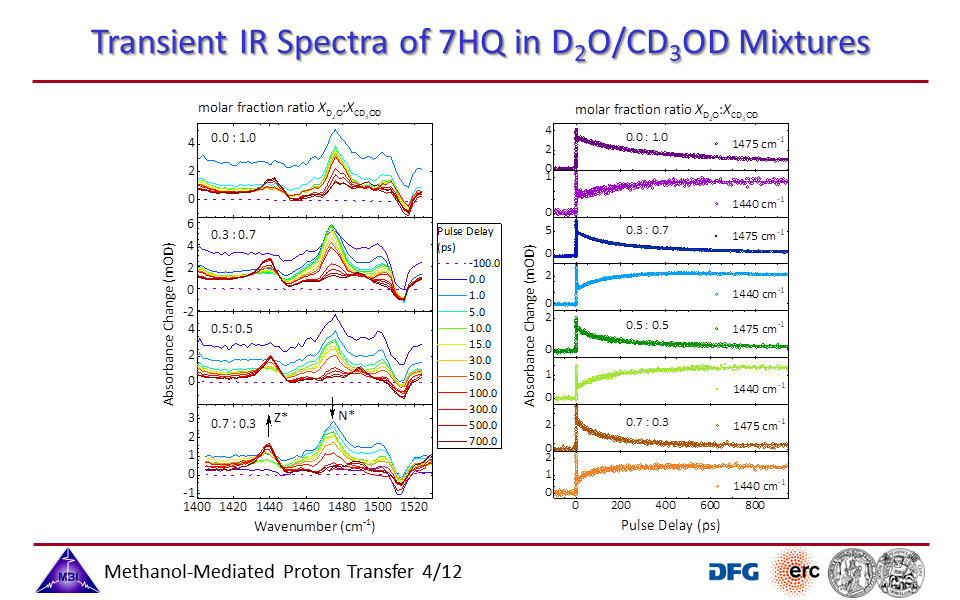
3.4. Transient UV/IR spectra monitoring the N* to Z* tautomerization reaction as a function of [CD3OD]:[D2O] molar fraction ratio.
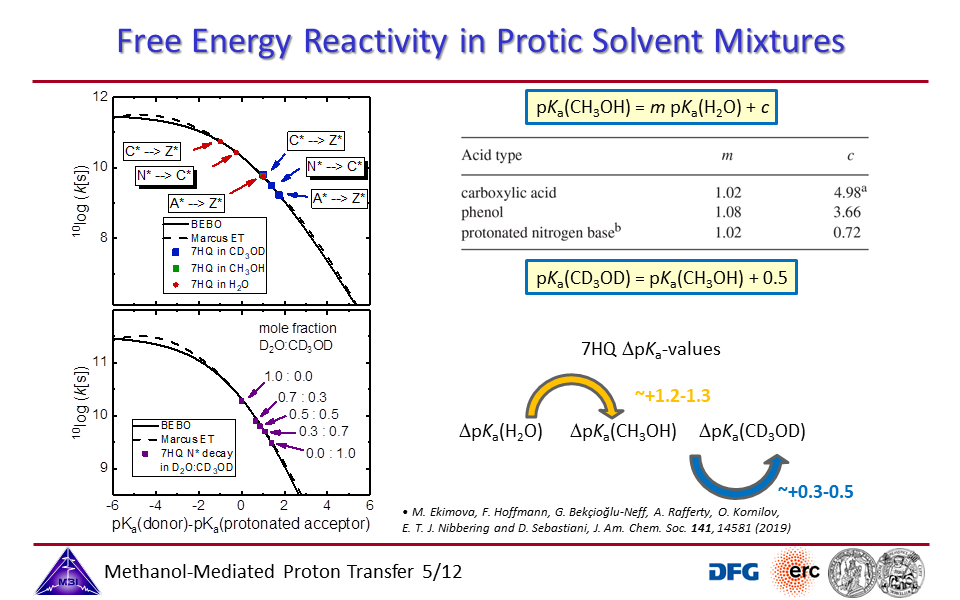
3.5. Free energy - reactivity relationship for acid-base proton exchange in protic solvents.
3.6. Solvent bridge vs. solvent wire pictures for different water/methanol mixing ratios.
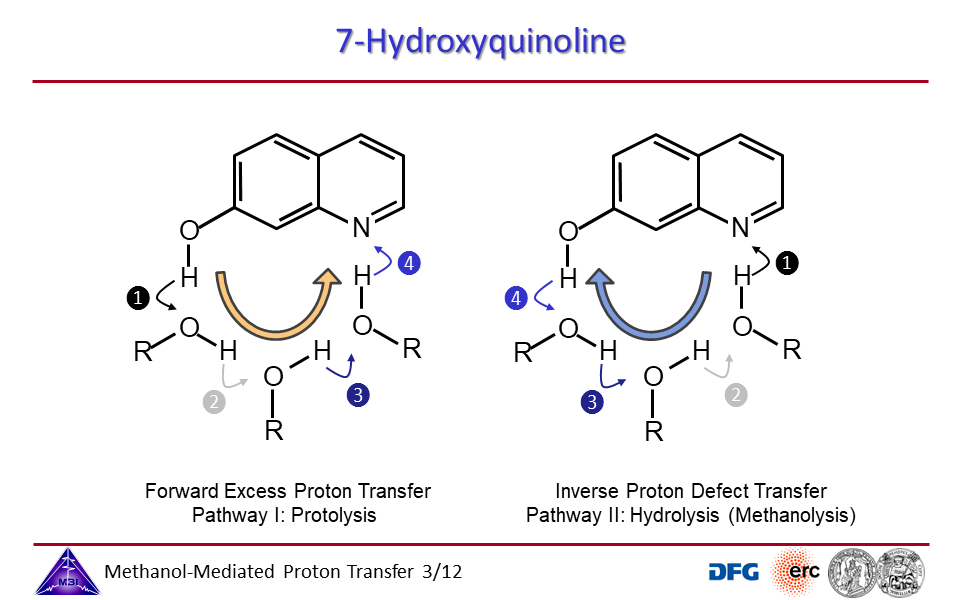
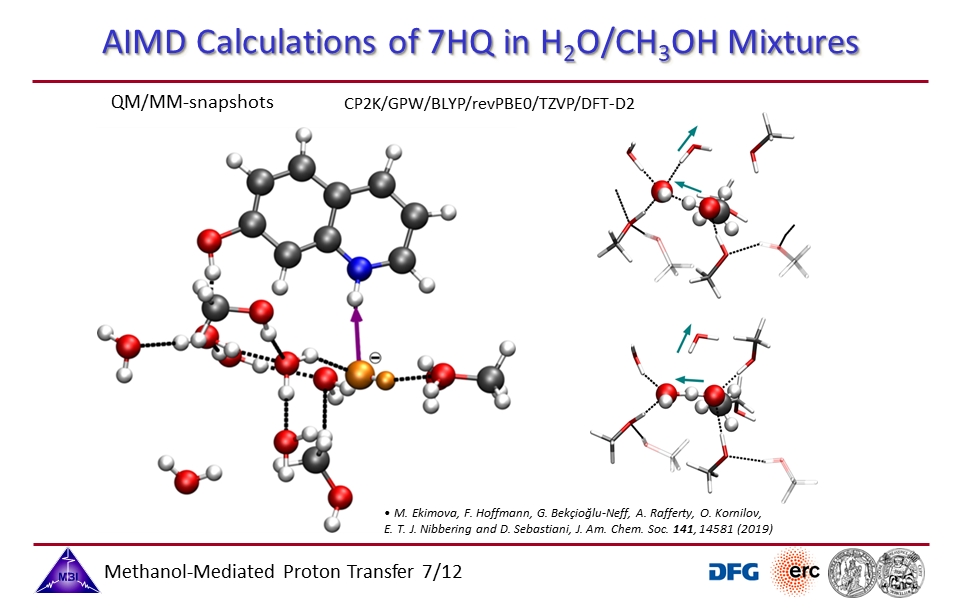
3.7. Ab initio molecular dynamics simulations of proton exchange from quinoline N-site to hydroxyl OH-site in 7-hydroxyquinoline.
3.8. Steering reaction pathways by additives: formate or imidazole.
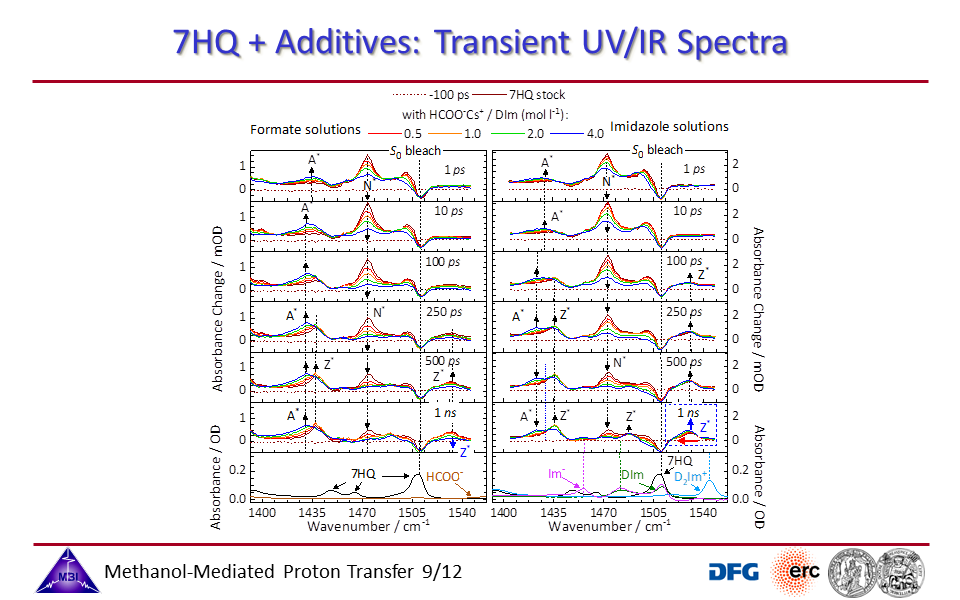
3.9. Transient UV/IR spectra of 7HQ with additives.
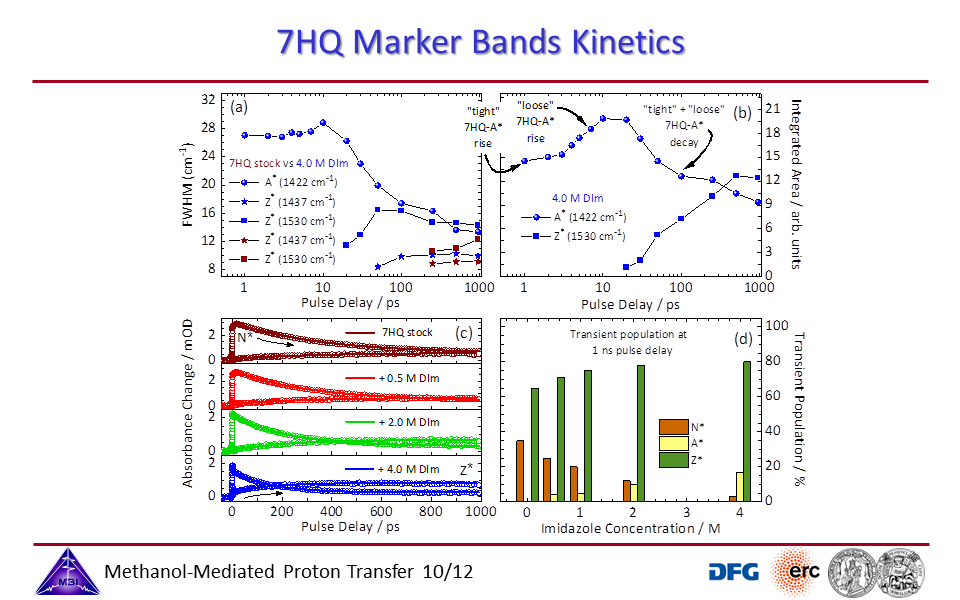
3.10. Transient kinetics of 7HQ with additives.
3.11. "Tight" and "Loose" reaction pairs of 7HQ + formate anion.
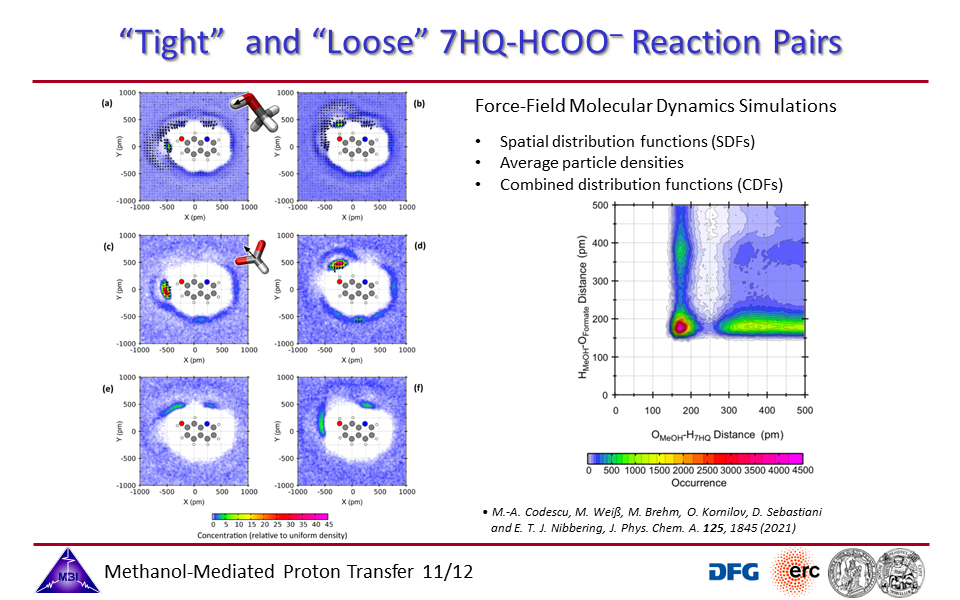
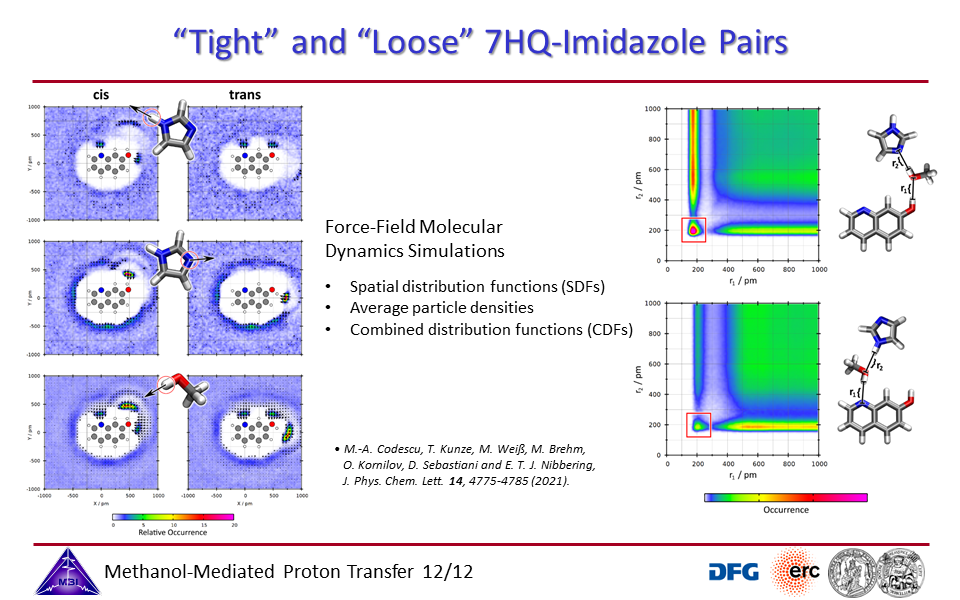
3.12. "Tight" and "Loose" reaction pairs of 7HQ + imidazole.