3.1 Dynamics of Condensed Phase Molecular Systems
Project coordinators: E. T. J. Nibbering , O. KornilovPhase 4 (2010-2013): Ultrafast dynamics of hydrated DNA oligomers, biomimetic base-pair systems and hydrated phospholipid self-assemblies
The people involved in Phase 4:
Łukasz Szyc, Ming Yang, Rene Costard, Christian Greve, Henk Fidder, Benjamin Koeppe, Ismael A. Heisler, Erik T. J. Nibbering, Thomas Elsaesser
National and international collaboration: Katharina Böttger,† Friedrich Temps,† Nancy E. Levinger,* Nicholas K. Preketes,# Shaul Mukamel#
†: Institut für Physikalische Chemie, Christian-Albrechts-Universität zu Kiel, Olshausenstrasse 40, D-24098 Kiel, Germany
*: Department of Chemistry, Colorado State University, Fort Collins, Colorado 80523-1872, United States of America
#: Department of Chemistry, University of California at Irvine, Irvine, California, USA
Hydrogen bonding of hydrated biomolecular and biomimetic systems is investigated with ultrafast vibrational spectroscopy. Hydration of biomolecules is addressed on the femto- to picosecond time scale by probing vibrational marker modes sensitive to molecular motions, energy exchange, and structural fluctuations of the hydration shell. Using artificial DNA oligomers as a model system and implementing a technique which allows for femtosecond measurements at a controlled variable hydration level, femtosecond 2D infrared and pump-probe spectroscopy is applied to provide new insight into hydration processes. Experiments on DNA oligomers and base pairs in solution addresses vibrational dynamics within the complementary hydrogen bonding structural motifs of base pairing, giving detailed information on the coupling and relaxation behaviour of the N-H stretching oscillators in these base pairs. The vibrational dynamics of phosphate groups in the DNA backbone and their energy exchange with the water shell have been determined for the first time, demonstrating that the surrounding water layers represent the preferential heat sink for excess energy released in DNA relaxation processes. These findings are complemented and confirmed by measurements on water pools nano-confined in reverse micelle structures with phosphate head groups.
Phase 4 (2010-2013):
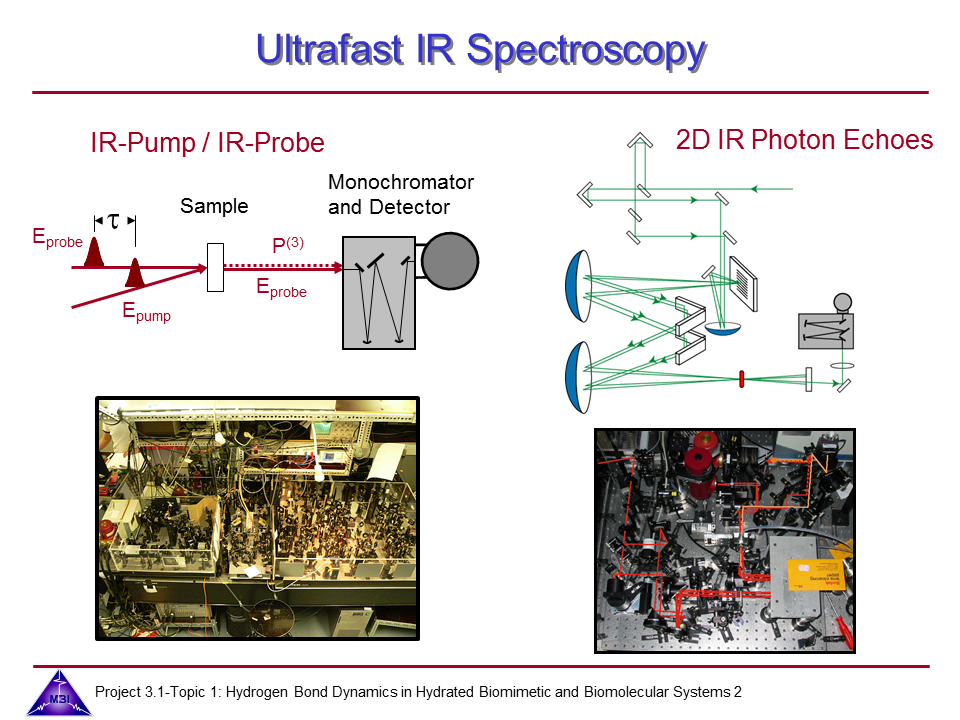
In the fourth period we expand our investigation on the vibrational couplings of hydrogen bonds in DNA oligomer films as well as intermolecular hydrogen bonded biomimetic systems, such as guanosine-cytidine or adenosine-thymidine base pairs in weakly polar solution. Hydration phenomena in DNA oligomers as well as in phospholipid self-assemblies are subject of this research. Implementation of 2D-IR photon echo techniques operating at different frequencies enables, together with two-colour IR-pump-IR-probe measurements, the in-depth exploration of ultrafast dynamics of local hydration interactions.
4-1 Vibrational dynamics in hydrated DNA oligomers, and biomimetic hydrogen-bonded base-pair systems
4-2 Ultrafast dynamics in hydrated phospholipid self-assemblies
Strategic approach
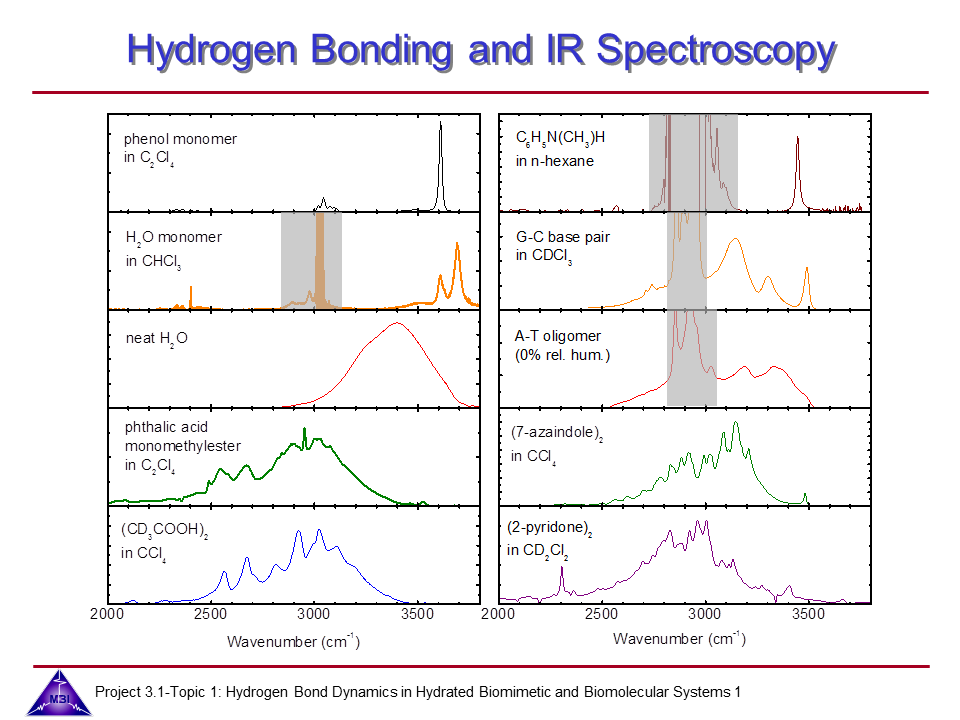
1. We investigate the structural and dynamical aspects of hydrogen bonds by determination of the coherent vibrational response of intra- and intermolecular modes that function as probe of these hydrogen bonds (grey shaded areas indicate C-H stretching modes of side groups, counter ions and/or solvent). In particular the O-H stretching and bending modes of water, the N-H stretching oscillators of DNA base pairs and the asymmetric (P-O2) stretching modes are extremely sensitive to the strength and dynamical fluctuations exerted on the hydrogen bonds, as being caused by the substantial anharmonic couplings of these modes with other fingerprint vibrations and with the low-frequency hydrogen bond modes. This is exemplified by the pronounced frequency red-shift and line broadening of the IR-active vibrational transitions of the O-H and N-H stretching modes shown in the graph.
Vibrational dynamics in hydrated DNA oligomers, and biomimetic hydrogen-bonded base-pair systems
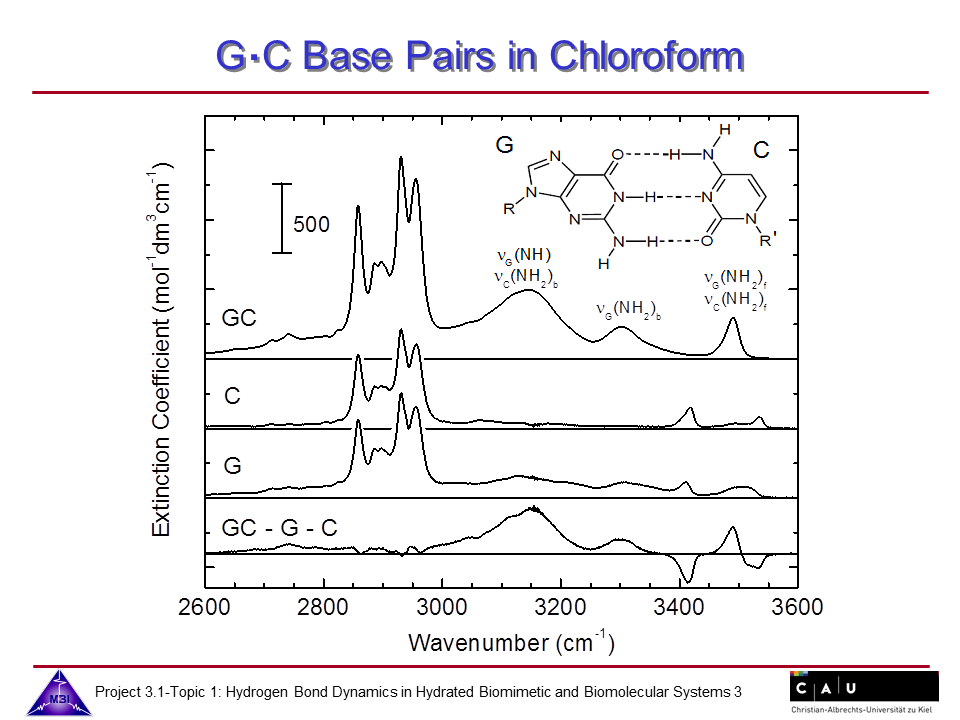
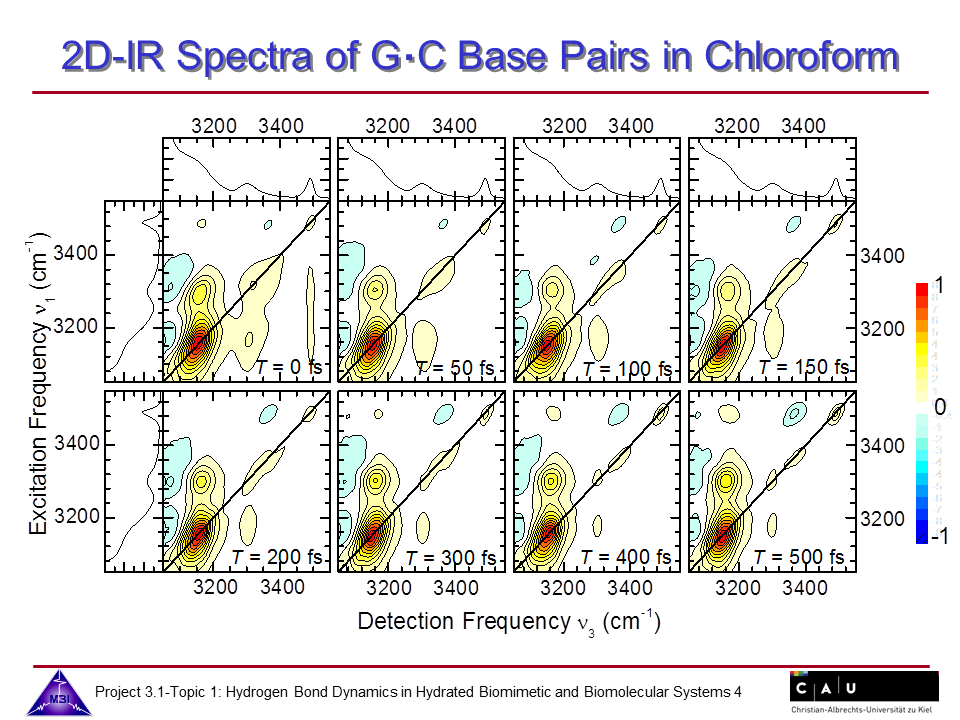
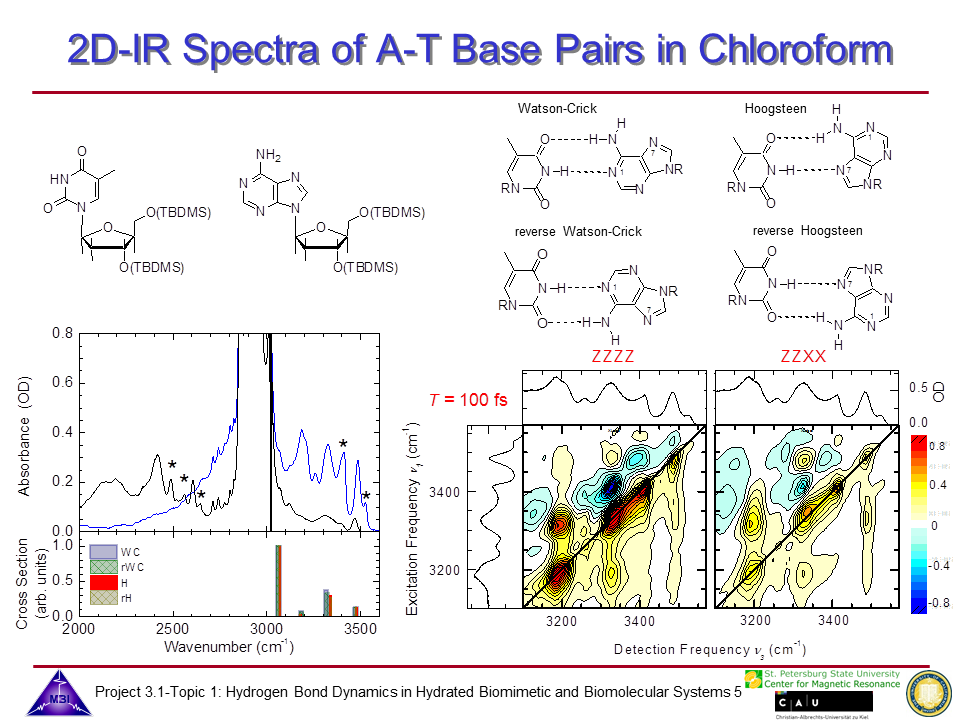
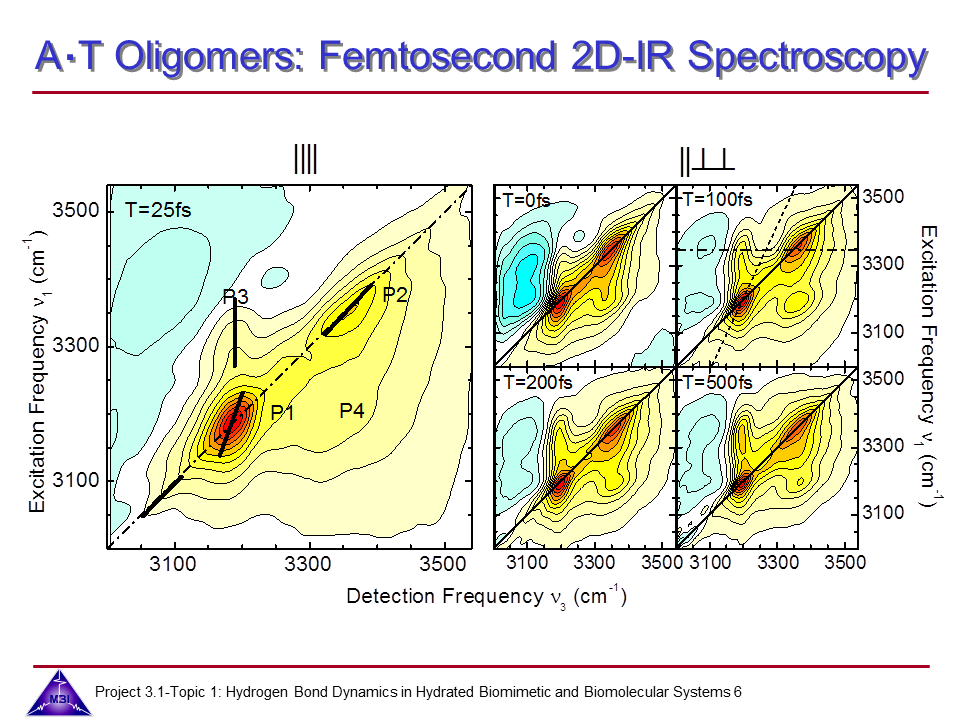
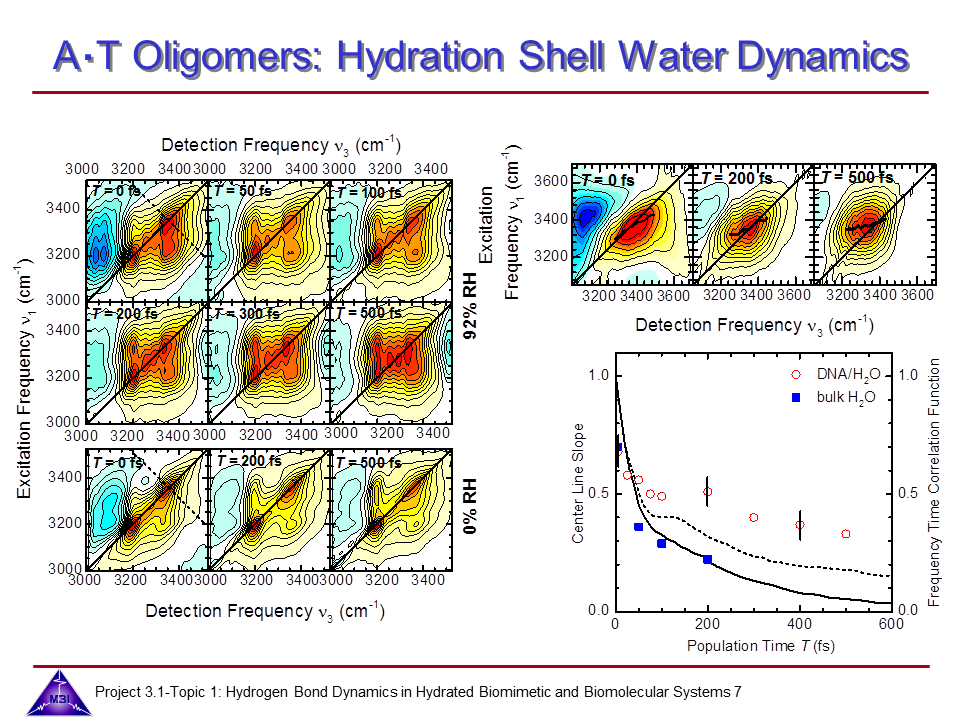
We continue our research on hydrogen bond dynamics in hydrated DNA base pairs. Dynamics and couplings of N-H stretching modes in guanosine-cytidine base pairs in chloroform solution, and in adenine-thymine in 23 oligomer Watson-Crick double helix DNA films are investigated with 2D-IR photon echo spectroscopy. Hydration shell water dynamics is probed with 2D-IR spectroscopy of the O-H stretching mode.
6. 2D-IR photon echo spectra measured for AT oligomer at 0 % relative humidity, measured for parallel linear polarizations of the 4 pulses (left), and perpendicular polarization of pulses 3 and 4 compared to pulses 1 and 2 (right), with which vibrational couplings, energy transfer and spectral diffusion dynamics of the N-H stretching manifold of AT oligomer can be grasped.
7. 2D-IR photon echo spectra measured for AT oligomer at 92 % relative humidity, and compared to the 0% relative humidity case, all measured with IR pusles tuned at 3250 cm-1. On the right 2D-IR spectra are shown for pusles tuned at 3400 cm-1. All data were recorded for perpendicular polarization of pulses 3 and 4 comapred to those of pulses 1 and 2. Centre of line slopes determined for the hydrated DNA case are compared to those obtained in bulk water, and to MD calculations with and without resonant energy transfer (taken from T.l.C. Jansen et al., J. Chem. Phys. 132 (2010) 224503.
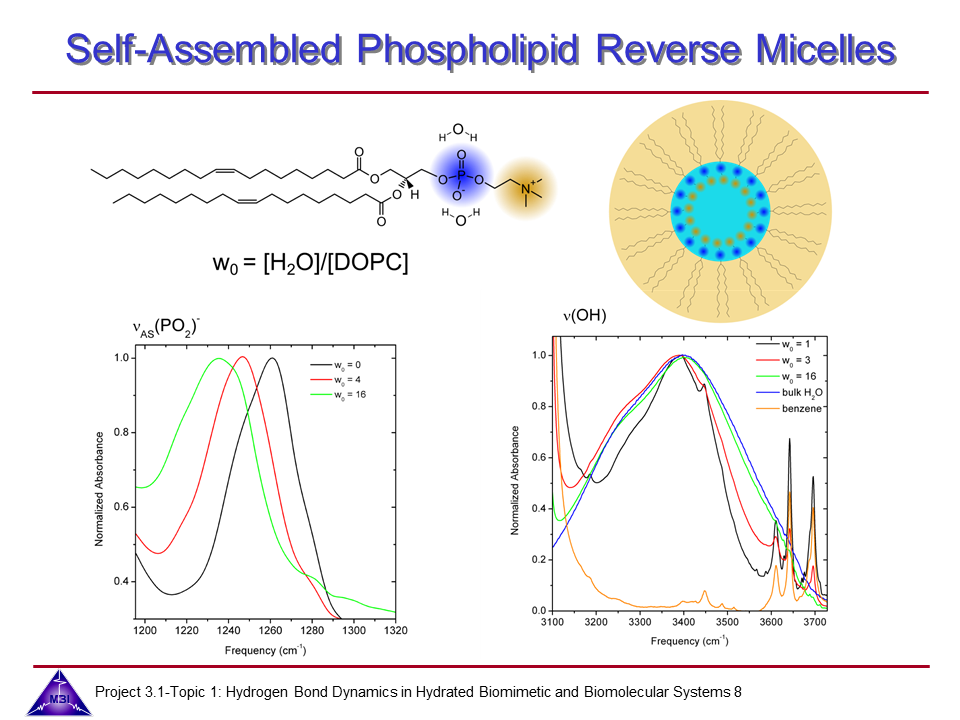
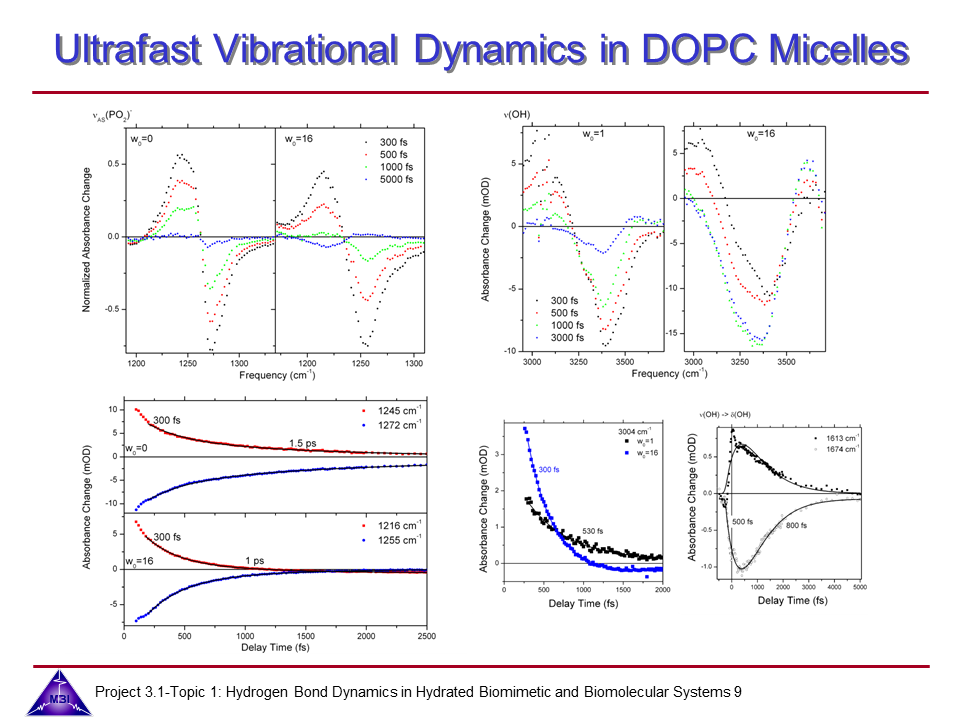
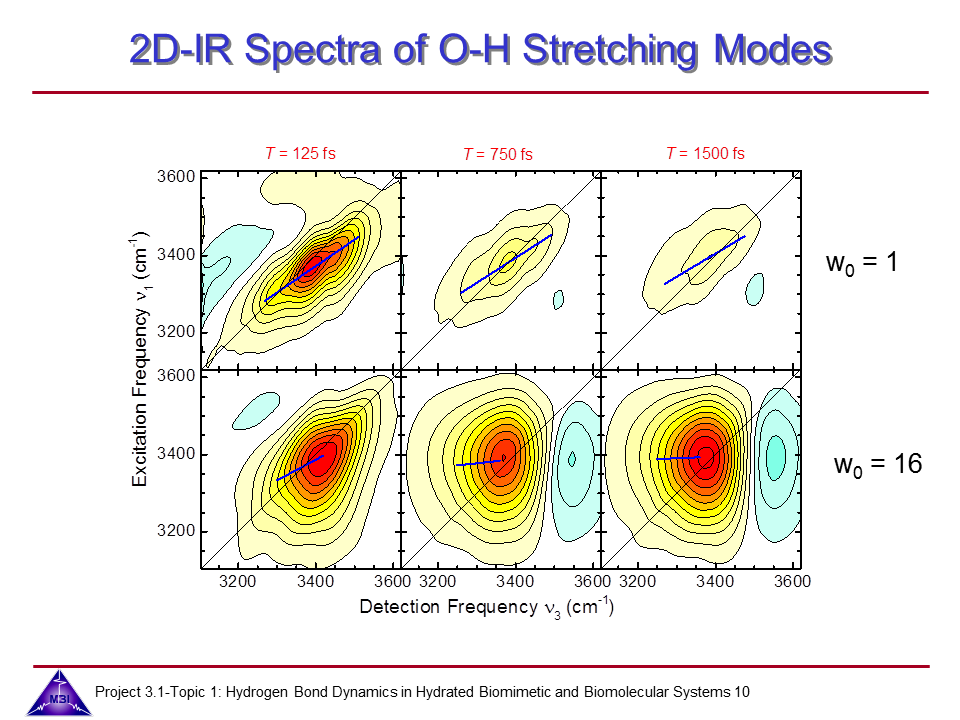
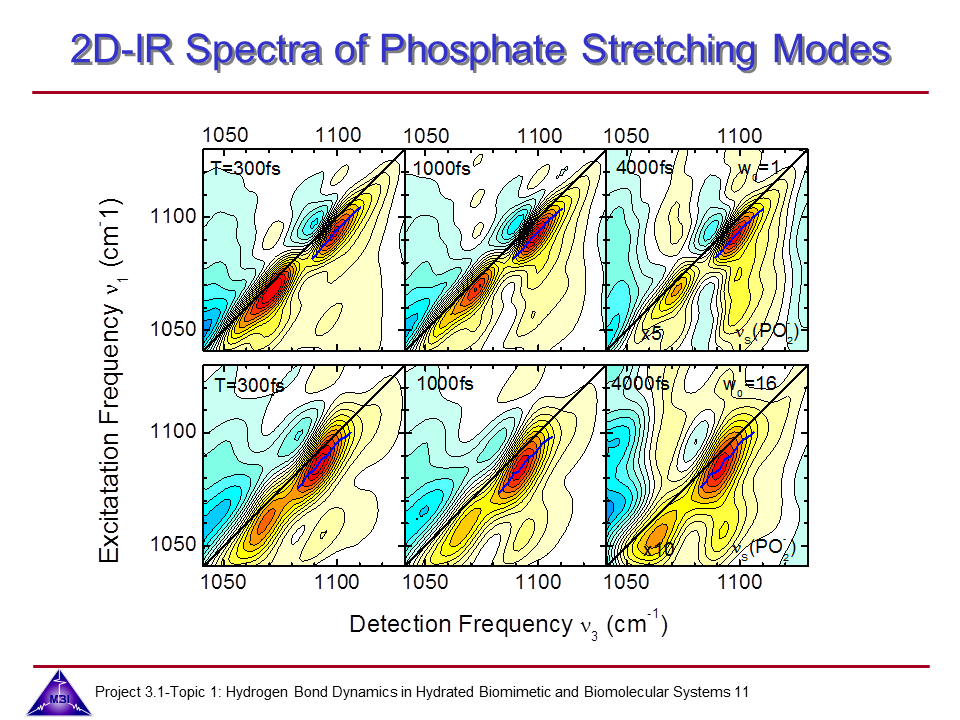
Ultrafast dynamics of hydrated phospholipid self-assemblies
Lipid molecules form a critical part of membranes found in all living systems. Self-assembly of these molecules into organized bilayers provides a framework that delineates cellular and subcellular compartments. Model lipid bilayers have been explored to infer information about structure and function of biological membranes. We investigate the vibrational dynamics of dioleoylphosphatidylcholine (DOPC) molecules self-assembled into phospholipid reverse micelles as a model system for phosphate–water interactions. The interaction of water molecules with ionic phosphate groups is of particular interest as phosphate groups are primary hydration sites in membranes and in biomolecules such as DNA and RNA.
9. One- and two-colour IR-pump-IR-probe spectroscopy monitoring the asymmetric (P-O2) stretching mode of DOPC and the O-H stretching mode of nanoconfined water. Two-colour pump-probe spectroscopy also demonstrates the vibrational energy flow of O-H stretching excitations to distribute via the water bending mode.