3.1 Dynamics of Condensed Phase Molecular Systems
Project coordinators: E. T. J. Nibbering , O. KornilovPhase 3 (2006-2010): Ultrafast dynamics of hydrated adenine-thymine DNA oligomers, biomimetic base-pair systems and temperature jump of neat liquid water probed at the O K-edge
The people involved in Phase 3:
Lukasz Szyc, Ming Yang, Jason. R. Dwyer, Jens Dreyer, Erik T. J. Nibbering, Thomas Elsaesser
Collaboration within Berlin scientific arena: Milena Petkovic,† Oliver Kühn,† Gianina Gavrila,º Kai Godehusen,º Christian Weniger,º Philippe Wernet,º Wolfgang Eberhardt,º Jing Guo,+ Peter M. Tolstoy,+Hans-Heinrich Limbach+
†: Through SFB-450 (Teilprojekt C1): Freie Universität Berlin, Institut für Chemie, Physikalische und Theoretische Chemie, Takustrasse 3, D-14195 Berlin, Germany
º: Ultrafast X-Ray Science Group, BESSY, Berlin, Germany
+: Institut für Chemie und Biochemie, Freie Universität Berlin, Takustrasse 3, D-14195 Berlin, Germany
International collaboration: Barry D. Bruner,* Michael L. Cowan,* Darren Kraemer,* Alexander Paarmann,* Jason R. Dwyer,* Brige Chugh,* Maher Harb,* R. J. Dwayne Miller ,* Shaul Mukamel,# Boguslawa Czarnik-Matusewicz§
*: Department of Chemistry and Physics, University of Toronto, 80 St. George Street, Toronto, Ontario, Canada, M5S 3H6
#: Department of Chemistry, University of California at Irvine, Irvine, California, USA
§: Faculty of Chemistry, University of Wroclaw, F. Joliot-Curie 14, 50-383 Wroclaw, Poland
Phase 3 (2006-2010)
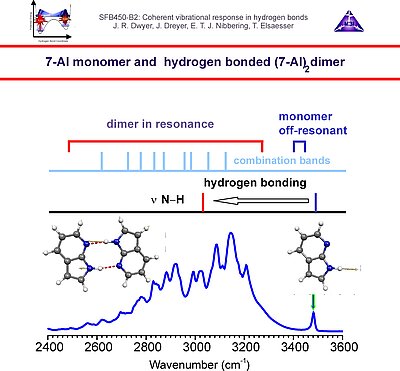
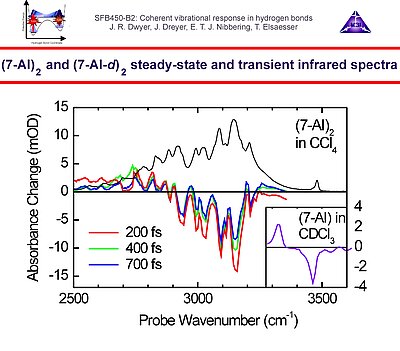
In the third period we investigate the vibrational couplings of hydrogen bonds in DNA oligomers as well as intermolecular hydrogen bonded biomimetic systems, such as 7-azaindole dimer. Here the couplings of the N-H stretching vibrations with the fingerprint modes, as well as with the low-frequency hydrogen bond modes playa key role in the vibrational dynamics. In addition we explore the couplings of the DNA oligomers with O-H stretching vibrations of solvation shell water molecules. A novel line of research is the investigation of the structural dynamics upon vibrational energy redistribution and relaxation of neat liquid water through inspection of the oxygen K-edge using ultrafast X-ray spectroscopy with high temporal resolution.
3-1 Vibrational dynamics in hydrated adenine-thymine DNA oligomers, and biomimetic hydrogen-bonded base-pair systems
3-2 Ultrafast response of the oxygen K-edge of neat liquid water upon a temperature jump
Strategic approach
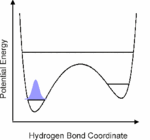
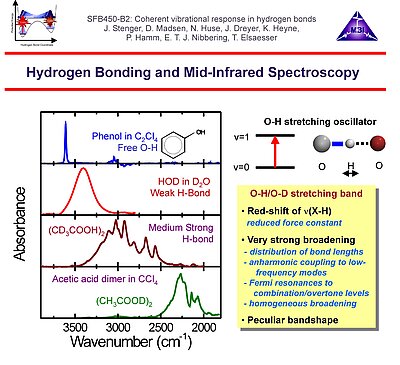
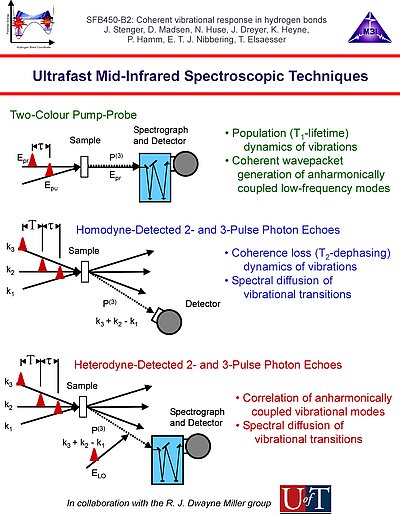
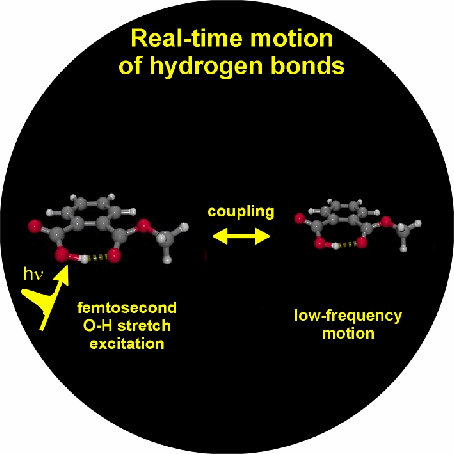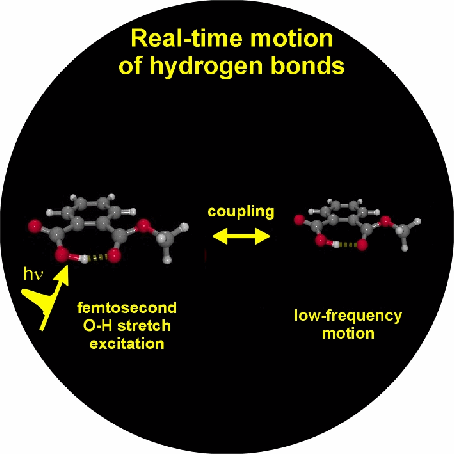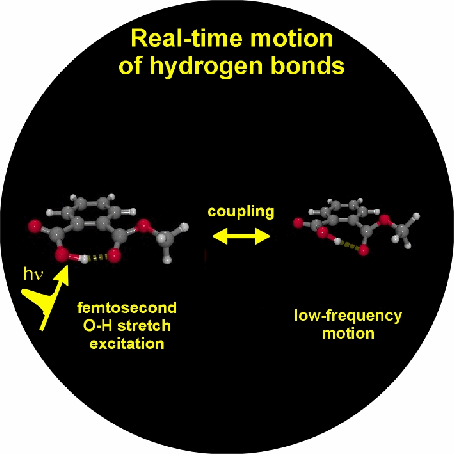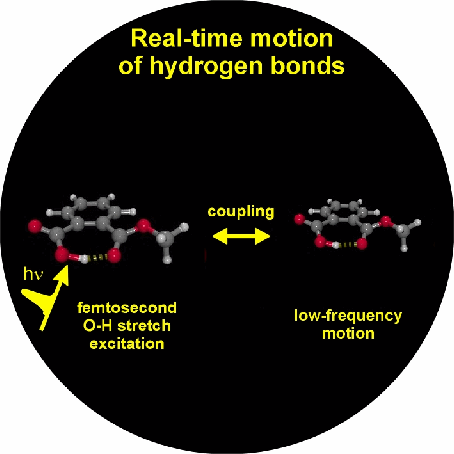
1. We investigate the structural and dynamical aspects of hydrogen bond networks by determination of the coherent vibrational response of intra- and intermolecular modes that function as probe of these hydrogen bonds. In particular the O-H stretching and bending modes are extremely sensitive to the strength and dynamical fluctuations exerted on the hydrogen bonds, as being caused by the substantial anharmonic couplings of these modes with other fingerprint vibrations and with the low-frequency hydrogen bond modes. This is exemplified by the pronounced frequency red-shift and line broadening of the IR-active vibrational transitions of the O-H stretching modes.
Vibrational dynamics in hydrated adenine-thymine DNA oligomers, and biomimetic hydrogen-bonded base-pair systems
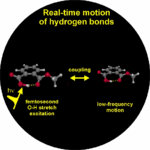
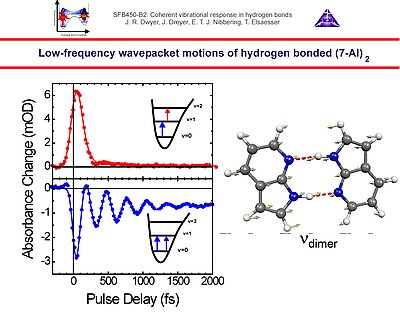
3. Vibrational perspective of hydrogen bonds: typically a strong anharmonic coupling exists between the O-H/O-D stretching and low-frequency modes that modulate the hydrogen bond distance. For this set of coupled oscillators this has the consequence that excitation of the O-H/O-D stretching mode includes excitation of these low-frequency modes, in a similar fashion as electronic excitation of molecules is accompanied by vibrational excitation. Ultrafast excitation of the O-H/O-D stretching mode in the mid-infrared thus leads to coherent wave packet motions of these low-frequency modes.
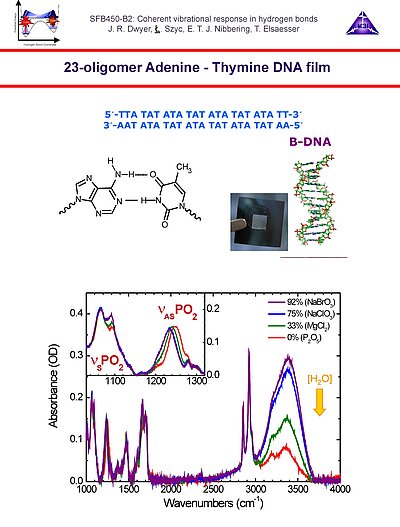
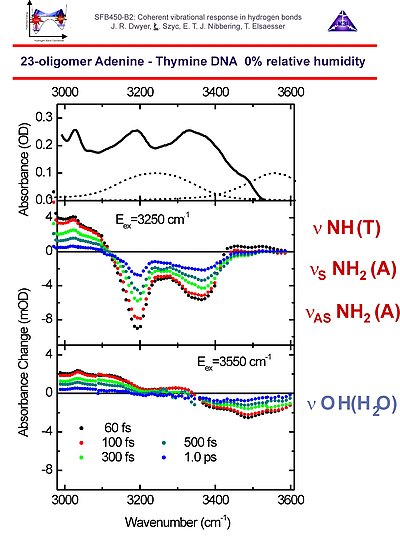
7. We use oligomers with 23 adenine-thymine base pairs with surfactant counterions to prepare optcally transparent DNA films with a water content controlled by varying the relative humidity in a specially designed sample holder. The IR spectra as function of relative humidity show pronounced humidity-dependent spectral features in the N-H/O-H stretching and in the symmetric and asymmetric phosphate stretching regions.
8. In the case of 0% relative humidity, with an average of 2 water molecules per base pair, the spectral substructure in the steady state spectrum (upper panel) can be dissected into bleach features due to the symmetric and asymmetric N-H stretching modes of adenine and the N-H sretching mode of thymine (central panel), and in a broad featureless component due to the O-H stretching mode of the residual water (lower panel). All these spectral features do not exhibit spectral diffusion up to several picoseconds, indicative of well-defined geometries on these time scales.
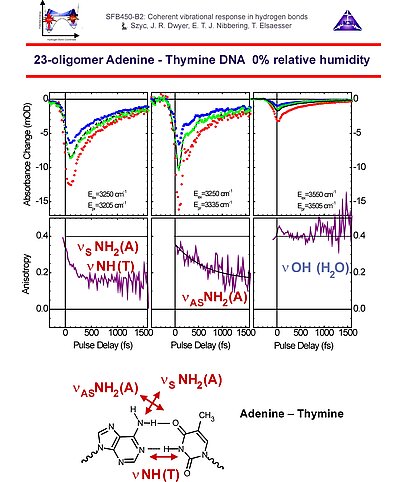
9. With polarization-sensitive pump-probe measurements the anisotropy decay curves can be determined. From these results it follows that the 3200 cm-1 bleach results from the symmetric N-H stretching of adenine and the N-H stretching of thymine exhibiting ultrafast excitation transfer. The 3350 cm-1 bleach component indicates the asymmetric N-H stretching transition of adenine, having smaller couplings with the other N-H stretching modes in the AT base pairs. The O-H stretching band of water around 3550 cm-1 does not show any anisotropy loss, in accordance with a location near the phosphate ionic heads, with no rotational diffusion occuring on time scales up to several picoseconds.
Ultrafast response of the oxygen K-edge of neat liquid water upon a temperature jump
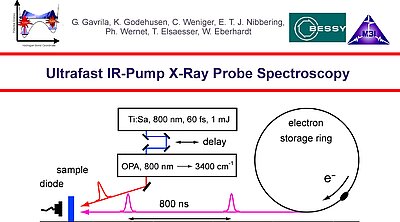
10. Ultrafast IR-pump X-ray probe spectroscopy using an Ti:sapphire amplified laser system and parametric frequency conversion to generate femtosecond IR pump pulses and the Bessy storage ring to generate soft-X-ray pulses used for probing x-ray absorption near edge and extended absorption fine structure.
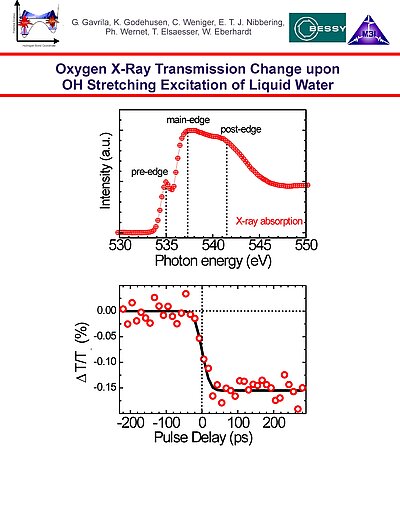
11. X-ray spectrum of th oxygen K edge of liquid water, showing pre-edge, main-edge and post-edge features indicative of the hydrogen bonding network structure. Transient X-ray transmission change at the pre-edge, upon IR-pumping of the O-H stretching intramolecular mode of liquid water, resulting in an ultrafast temperature jump with a decrease of the average hydrogen bonding coordination number of the water molecules.