Biochemical processes take place predominantly in an aqueous environment. Certain groups of a biomolecule are embedded in a shell of water molecules, i. hydrated. The water envelope stabilizes the biomolecular structure and allows the exchange of energy between the biomolecule and the environment. Examples of such systems are the DNA double helix, the carrier of genetic information, in an aqueous environment, and the phospholipid outer membrane of living cells. The molecular mechanisms that determine the speed and efficiency of the energy exchange between the biomolecule and the water envelope are only beginning to be understood and are therefore the subject of current research.
Small and efficient - water nanodroplets cool biomolecules extremely fast
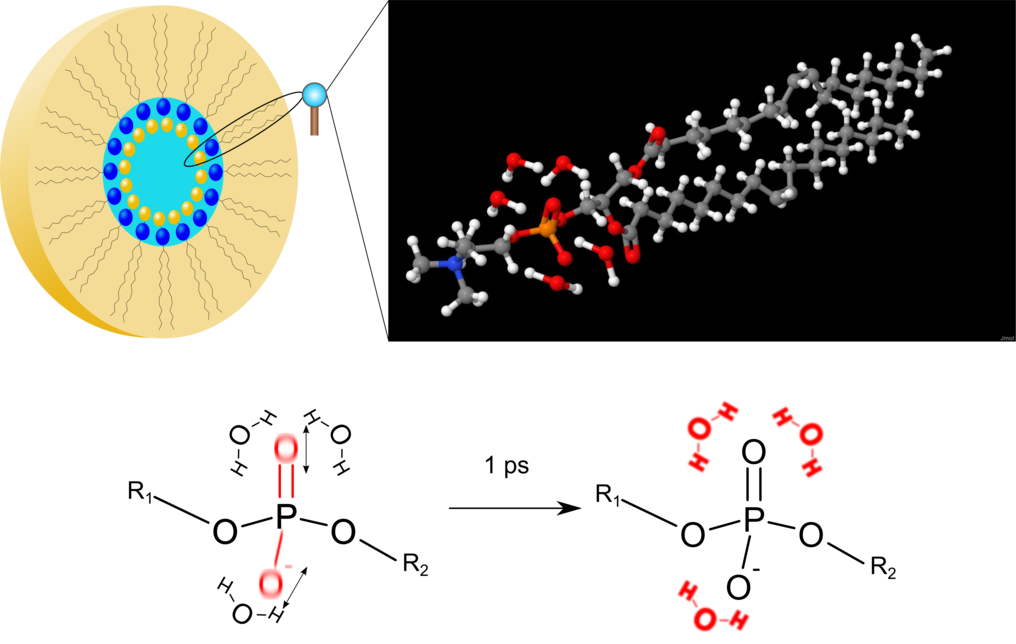
Fig. 1 Top left: Schematic representation of an inverse micelle consisting of phospholipid molecules. The phosphate groups of the lipid molecules (blue spheres) are located on the inner surface of the micelle. Water molecules are inside the micelle. Upper right: Enlarged view of the structure of a phospholipid molecule. Oxygen atoms are shown in red, hydrogen atoms in white, carbon atoms in gray, the nitrogen atom in blue and the phosphorus atom in orange. The angled water molecules are located in the vicinity of the phosphate group (PO4). Below: Scheme of energy transfer. In the experiments, first the (asymmetric) stretching vibration of the phosphate group is excited (red oxygen atoms O). After the decay of the vibration excitation, the released energy is transferred within a picosecond to the surrounding water envelope (red water molecules H2O).
Researchers at the Max Born Institute have now shown that minute water "droplets" in the vicinity of a lipid molecule have energy transfer in the time domain below 1 ps, i. in less than 1 millionth of a millionth of a second. As René Costard, Christian Greve, Ismael Heisler, and Thomas Elsässer report in the latest issue of the Journal of Physical Chemistry Letters (Volume 3, page 3646, 2012), 3 water molecules coupled to the phosphate group of the lipid are sufficient to release vibrational energy from the lipid efficiently transfer and transform into thermal energy of the water envelope. The water jacket is heated by 10 to 20 ° C. The thermal energy is mainly in tilting movements of the water molecules, so-called. Librationen, and leads to a weakening of the interaction between the water molecules, the so-called. Hydrogen bonds. The molecular structure of the water envelope remains almost unchanged on the time scale of the energy transfer. This extremely efficient mechanism also allows for the transmission of larger amounts of energy and can thus protect the lipid molecule from damage to its structure by overheating.

Fig. 2 Two-dimensional infrared spectra of the OH stretching vibration of a water envelope consisting of 3 water molecules per phosphate group. The left picture shows the spectrum of excited OH stretching vibrations of the water jacket at the time 0.125 ps. The signal is shown as a yellow-red contour as a function of the excitation and the detection frequency. The right spectrum was recorded after 1.5 ps and shows the characteristic signal of a heated water jacket. The additional contribution at high detection frequencies (blue contour) is due to the weakening of the interaction between water molecules in the heated shell.
The experiments investigated a phospholipid model system consisting of DOPC molecules (Fig. 1). These molecules are arranged as so-called inverse micelles, in the interior of which the phosphate groups (PO4) of the lipid molecules are hydrated. The water content can be changed within wide limits. To investigate the energy transfer, light pulses of about 0.1 ps were used to excite either a phosphate oscillation of the lipid or the OH stretching vibration of water molecules. Both vibrations decay in fractions of a picosecond and release the released energy to the water jacket. This transfer and redistribution process was monitored by measuring transient two-dimensional vibrational spectra of the OH stretching vibration of the water (Fig. 2). The weakening of the hydrogen bonds in the heated water envelope leads to a shift of the OH stretching vibration to higher frequencies. From the time-dependent change of these spectra, the dynamics of the energy transfer can be derived directly.