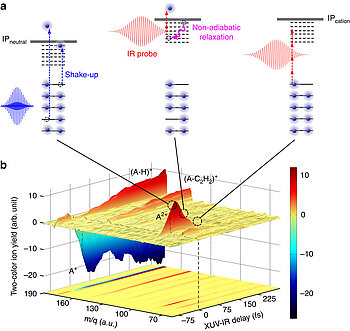
With the help of ultrafast UV lasers, the scientists were able to decode the dynamics of highly excited molecular states. Among the hydrocarbons, which are likely to be the carriers of the absorption bands, the polycyclic aromatic hydrocarbons were particularly promising. The presence of PAH / PAH molecules has previously been deduced in many astronomical objects, such as the interstellar matter clouds of our Milky Way galaxy, but even in ten-billion-year-old matter from the early days of the universe. However, astronomers also had doubts about the hypotheses because the lifetime of the unusual molecular states was unknown. The MBI researchers in collaboration with scientists from the University of Lyon, supported by theoretical calculations by scientists at the Universities of Leiden, Heidelberg and Hyderabad, have now shown that the lifetime of the electronic states of small to medium-sized PAHs coincides with the line widths be observed in the diffuse absorption bands.
In the experiments, a series of small to medium-sized PAH molecules (naphthalene, anthracene, pyrene, and tetracene, each containing several condensed aromatic rings) were ionized with an ultra-short ultra-violet laser pulse (XUV). The absorption of an XUV photon not only led to the removal of one of the electrons, but also to the electronic excitation of the resulting positively charged molecule ion. The lifetime of these excited cationic electronic states was measured by means of a time-delayed infrared laser pulse.