How much time does an atom take to absorb a photon and release an electron? And what if not one, but many photons are needed for the ionization? How much time would the absorption take by many photons? These questions lie at the core of attosecond spectroscopy, which aims to resolve electron motion on its natural time scale.
Ionization in strong infrared fields is often considered to be the tunneling of electrons through a potential barrier. The barrier is formed by the combination of the atomic potential, which binds the electron, and the electric field of the laser pulse, which pulls the electron away. Therefore, attosecond spectroscopy unexpectedly faces a nearly age-old and controversial question: how long does it take an electron to tunnel through a barrier?
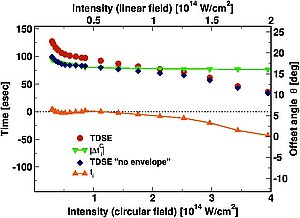
In the publication by Torlina et al. this question is pursued on the basis of the so-called Attouhr structure. Attohr uses the rotating electric field of a circularly polarized laser pulse as a pointer of the clock. One full turn of this hand lasts one laser period, approximately 2.6 fs for experiments with 800 nm pulses of a titanium: sapphire laser. The tunneling barrier also rotates with the rotating electric field. Therefore, electrons tunneling at different times tunnels in different directions. It is this connection between time and direction of the electron movement that enables Attouhr to measure times.