Solvation Dynamics of Excess Protons
- Ultrafast Motions and Fleeting Geometries in Proton Hydration [Science 357, 491 (2017)]
Basic processes in chemistry and biology involve protons in a water environment. Water structures accommodating protons and their motions have so far remained elusive. In collaboration with the group of Prof. T. Elsaesser, we have mapped fluctuating proton transfer motions via ultrafast vibrational spectroscopy and provide direct evidence that protons in liquid water are predominantly shared by two water molecules.
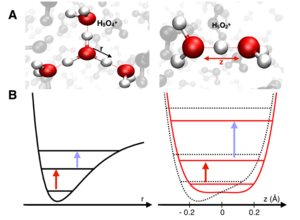
Protons in water are commonly described with the help of two limiting structures, in the Eigen complex (H9O4+), the proton is part of the central H3O+ ion surrounded by three water molecules. In the Zundel cation (H5O2+), the proton forms strong hydrogen bonds with two flanking water molecules. The potential energy surface of the proton are expected to be markedly different for the two limiting geometries: one expects an anharmonic single-minimum potential for the Eigen species and a double minimum potential for the Zundel species. In liquid water, such potentials are highly dynamic in nature and undergo very fast fluctuations due to thermal motions of surrounding water molecules and the proton. The femtosecond dynamics of proton motions were mapped via vibrational transitions between proton quantum states. The sophisticated method of 2D-IR spectroscopy reveals that the transition between excited quantum states (blue arrow) occurs at higher detection frequencies than the transition from the ground state (red arrow), giving the first direct evidence for the double-minimum character of the proton potential in the native aqueous environment.
- Fluctuating liquid structure induces ultrabroad infrared absorption: the hydrated proton on ultrafast time scales [Angew. Chem. Int. Ed. 55, 10600 (2016)]
Vibrational spectroscopy has helped to identify limiting molecular structures of hydrated protons such as the Eigen- and Zundel-cations. In particular the latter displays an extremely broad and unstructured infrared absorption, the so-called Zundel continuum. The mechanisms underlying the absorption continua have remained highly controversial.
For the particular model case H5O2+ in acetonitrile, we could dynamically dissect the Zundel continuum from the regular OH stretching and bending vibrations of the two water molecules. The different excitations show lifetimes below 60 fs, much shorter than the OH stretching and bending vibrations of neat water.
Our theoretical analysis of the results demonstrates that the extreme broadening of the infrared absorption is caused by motions of the inner proton exerted by the strong, rapidly fluctuating electrical fields that originate from the surrounding polar solvent molecules. The energy of proton motions along the proton transfer coordinate is strongly modulated by these external fields, resulting in a concomitant modulation of vibrational transition energies. On a time scale faster than 100 fs, the system explores a broad range of transition energies, leading to the observed extreme broadening of the infrared absorption. Vibrational overtones and combination tones with modes changing the distance between the two water molecules contribute as well.
This new view at the Zundel cation clearly goes beyond the many studies of gas phase cluster work on hydrated protons, where due to the low temperature conditions, the field fluctuations underlying the Zundel continuum mechanism do not occur. The results are of relevance for many dynamic aspects of hydrated protons, be it for proton transport in water, the infamous von Grotthuss mechanism, in hydrogen fuel cells, or biological systems functioning with proton translocation mechanisms.